
In an industry heavily focused on the minutiae of compliance and regulatory requirements, we felt it was important to take a step back and learn about the people behind it all: those on the front lines of TMF management.
What are the realities of TMF professionals in the clinical trial arena of 2023? What are their pain points, where do they need more support, and how do they feel? The results from this survey give a collective voice to those with an undeniable and direct impact on clinical trial success.
With this survey, we were able to surface some of the trends that are shaping the trajectory of our industry. These discoveries relate to everything that makes TMF professionals tick: inspection readiness, metrics, QC, and more. Certain findings were to be expected, but many others were surprising. Yet, despite the array of different topics touched upon in the survey, each piece of information it has produced is bound by a common thread: you won’t find it anywhere else.
Below, we share with you authentic, original insights and data collected from one of the largest gatherings of TMF professionals on the planet. Our goal is to educate by uncovering patterns of thought and create a community by facilitating the discussion on the evolving and ever-important role of the TMF.
Remember, a trial without a complete TMF, cannot be completed at all.
Table of Contents
- About this survey
- Report highlights
- Who were our survey respondents?
- Forms of TMF management
- Inspectors' expectations in 2023: Are they becoming too difficult to keep up with?
- The most cited impediments to implementing an eTMF system
- Implementing an eTMF system: What (do you think) is in it for you?
- TMF tardiness: What's preventing teams from filing in a timely manner?
- Expert tip: How to improve TMF culture across your organization
- Where TMF teams struggle the most when it comes to inspection readiness
- Beyond measure: The most difficult TMF metrics to track
- Quality qualms: What's slowing down QC?
- The people have spoken: Desired features and improvements to current TMF systems
- Let's continue the discussion
About this survey
Who: 245 TMF Professionals
What: TMF Pulse Check Survey
When: Data collected May 16 – May 22, 2023
Where: Global audience
Why: To keep a pulse on the realities of TMF professionals today in order to report on their most pressing challenges and opinions.
How: 11 qualitative and quantitative questions via Typeform
*Please note: The statements and data in this text are not intended to be all-encompassing generalizations about the TMF industry or sentiments around the TMF market at large. These are the perceptions of a small yet diversified cohort to which we are offering our unique interpretation. We understand that a larger or different sample may have yielded different results, however we are confident in the fascinating results that are brought forth and discussed in this report.
Report highlights
Contributor motivation is a red flag to monitor
People’s lack of engagement and motivation around the TMF are the greatest limiter to TMF success. Deep dive into the untapped role of TMF Culture.
Cost of implementing eTMF tech is a leading roadblock
The perceived financial unattainability of eTMF tech is the most cited reason for not switching to a fully electronic system. Learn which market segments dominate this view.
Inspector expectations are a mounting pressure point
There is a conviction amongst TMF professionals that inspector expectations are becoming too difficult to manage. Uncover the expert interpretation behind these sentiments.
Completeness is the troublemaker metric to watch
Completeness surfaces often as a recurring point of contention for TMF professionals. Discover where things are going wrong.
Who were our survey respondents?
Industry segments
- 33.9% – CRO
- 24% – Pharma
- 22.7% – Biotech
- 3.7% – Non-profit
- 3.3% – Medical device
- 12.4% – Other
Company sizes
- 28.6% – 1-100
- 26.1% – 100-500
- 11.4% – 500-1000
- 33.9% – > 1000
Forms of TMF management
- 66.1% – eTMF System
- 11.4% – I do not manage TMF content
- 9.8% – Hybrid
- 5.3% – Outsourced
- 4.5% – Paper TMF
- 2.9% – File share
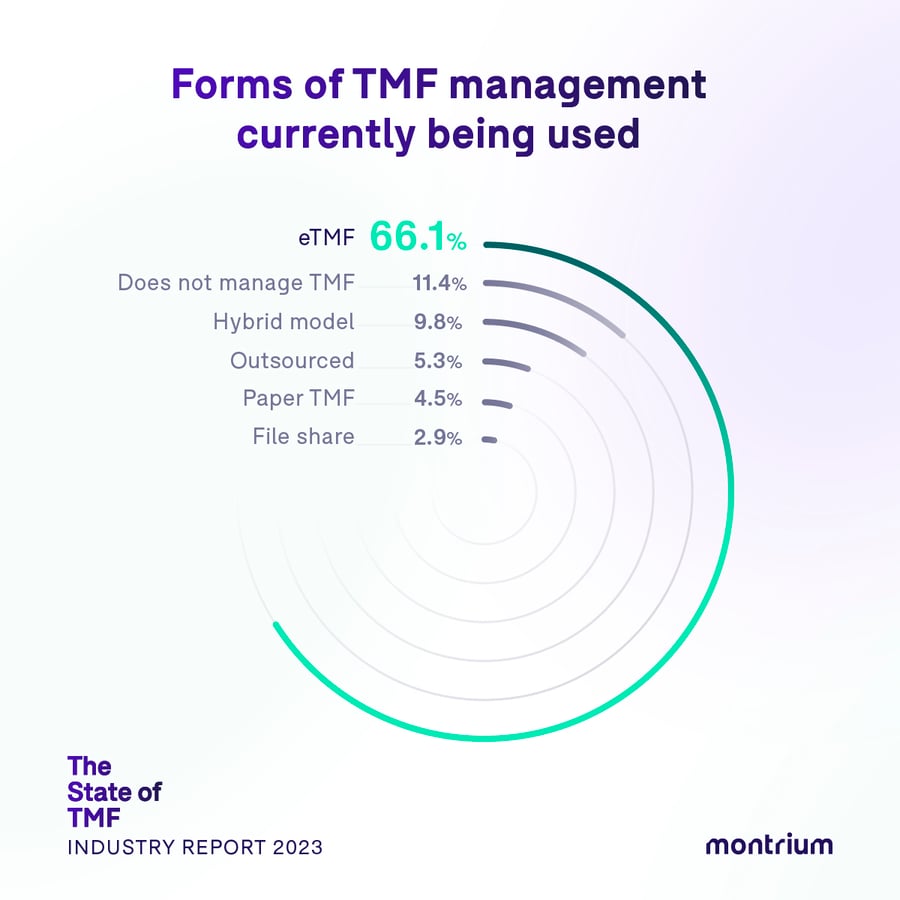
The electronic era
We were thrilled to see that 66% of survey respondents are currently using an electronic trial master file. This fact alone tells us that the industry shift away from paper is well underway.
Nearly 10% of survey respondents currently use a hybrid model, and while this isn't a massive number, it does reveal that there is still a pull towards paper, or rather a hesitancy to take the leap and move past it all together. A foot in both worlds. It's reassuring to see however that only 4.5% of survey respondents rely solely on a paper TMF, but we still question the rationale behind continuing what we believe to be a dated and restrictive method. Is it a mindset? Is it a lack of buy-in from the top? And what would it take to propel these companies into the electronic now of modern TMF management?
Inspectors’ expectations in 2023: are they becoming too difficult to keep up with?
- 51.4% – Neither agree nor disagree
- 26.5% – Agree
- 14.7% – Disagree
- 6.5% – Strongly agree
- 0.8% – Strongly disagree
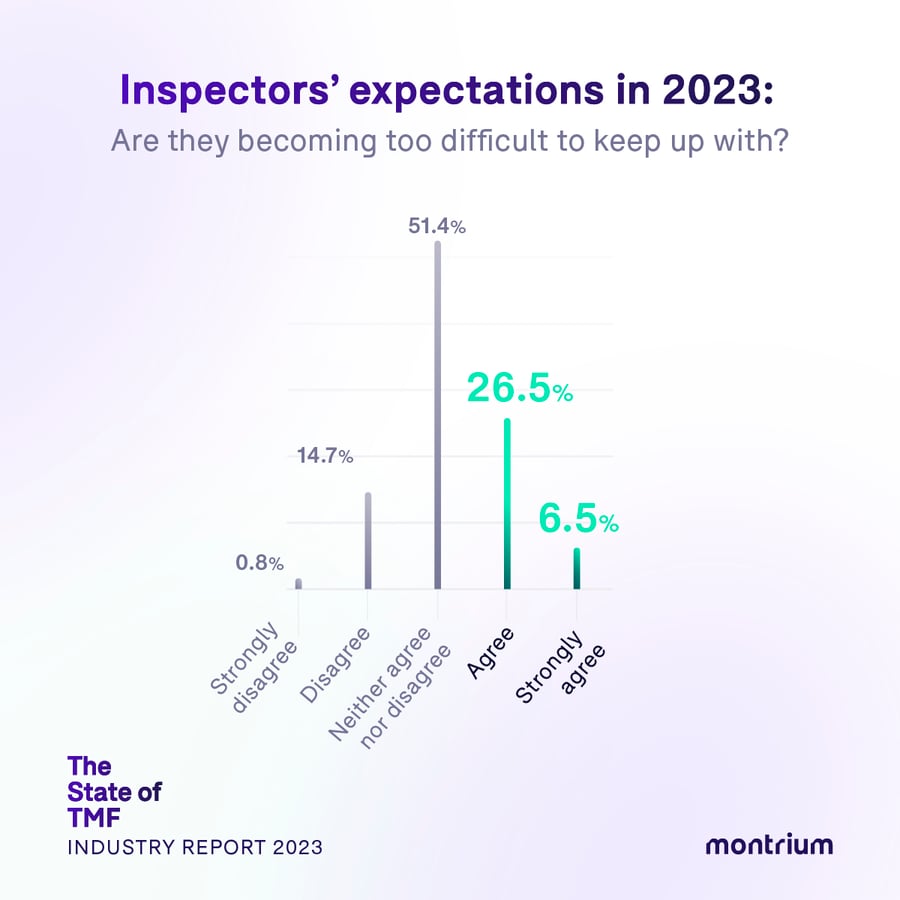
Great expectations
While just over 50% of respondents hold a neutral attitude towards an increased difficulty in inspector expectations, still 1/3 of respondents “agree” and “strongly agree” that expectations are becoming increasingly difficult. Christina Mantzioros, Head of Clinical Solutions Strategy at Montrium explains that “the scope of an inspection, i.e. what the inspector wants to look at now vs. before, has in fact become more challenging. Given electronic systems, there is obviously a lot more data available and whereas the regulations say that only final records (documents) are inspectable, in theory, inspectors want to see more than that now. For example, draft documents and all activities that took place on them. Not to mention, inspectors now expect access to audit trails and activity logs in readable formats, while many still lack an established periodic review process to ensure they are up to par. Evolving regulatory requirements is another reason—the industry was always slow in updating regulation in the past, but it seems like it's happening faster now, just because they have to keep up with the advances in technology.”
As the life science industry evolves and regulations continue to become more prolific and rigid, companies are forced to tighten their processes and assume a more “real time” approach to their TMF. This boils down to using it less as an archive, and instead as an active, data-driven lever to tell a complete, inspection-ready clinical trial story.
Easier access to the TMF
Tides are shifting. With the rise of remote and hybrid inspections, geographical barriers have become eliminated, allowing for quicker access to companies, meaning shorter notice for inspections and easier access to the TMF. The days of preparing for inspections at the last minute have sailed, forcing companies to be more on top of their TMF story as they go, and once again, demonstrating why “real time” completeness of the TMF is fundamental.
Our Head of eTMF Services, Donatella Ballerini, agrees that times are changing, as “inspectors are becoming more and more familiar with technology as well as the expectations around data integrity. The complexity of the environment is increasing, as are the number and intensity of guidelines and regulations. There are significant new rules for everyone, and often not enough direction for how to apply them properly, making it extremely challenging to be in compliance – to be prepared for inspectors.”
Finding
As we dig deeper, we see that 40% of companies not using a complete eTMF system “agree” or “strongly agree” that inspectors' expectations are becoming too difficult to keep up with, compared to only 17% of survey respondents who are currently using a full electronic system.
Our takeaway
The sentiment and perception that inspector expectations are becoming increasingly difficult is directly correlated to the type of TMF being used. While we have seen evolving expectations in the inspection landscape, we don’t believe inspector expectations are the sole culprit.
The stress and fallibility that comes with the taxing mental load of not using full electronic systems may be tainting the view of inspector expectations. The need to manually upload documents without access to key insights and data – to accurately grasp whether the TMF is complete or compliant - lends to the demandingness and stress of inspections all around. When processes and systems do not work in favour of inspection readiness, everything relating to inspections can feel “challenging”.
The most cited impediments to implementing an eTMF system: Why some aren’t taking the leap
- 32% – Cost of implementation
- 22% – Time to get up and running
- 21% – Getting management buy in
- 14% – System training
- 8% – Cost of subscription
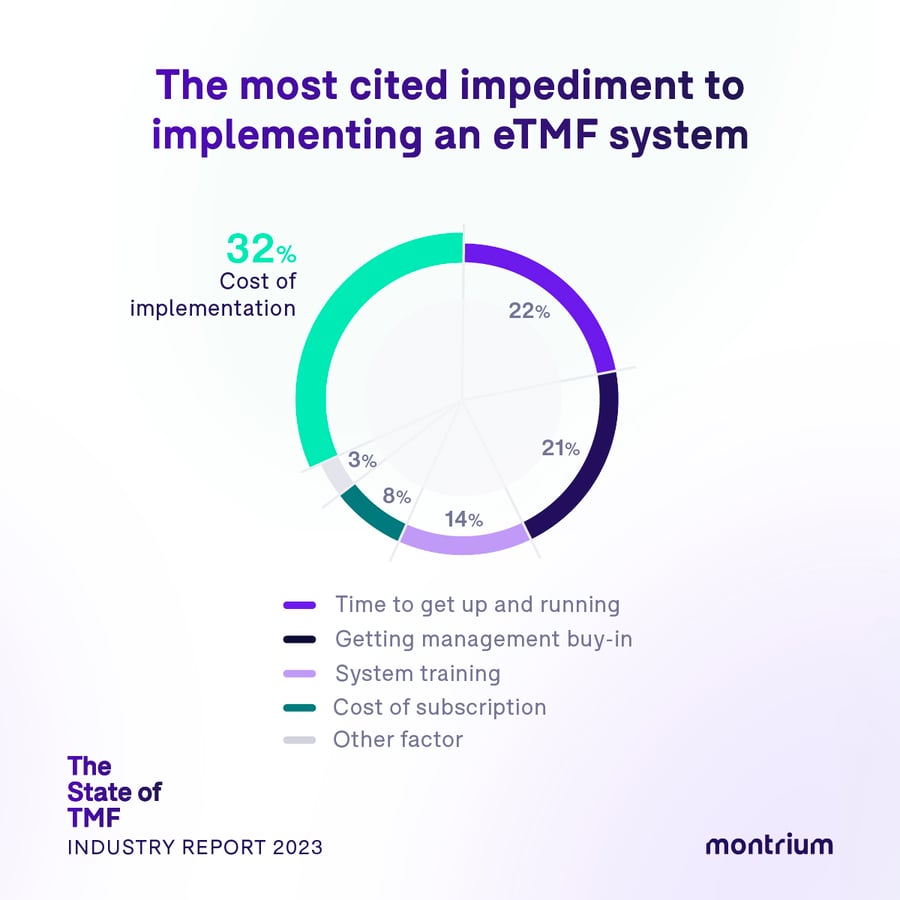
The great financial debate
The main impediment to making the full switch to an eTMF system is the “cost of implementation”, and when we dig deeper into our segmented data we see that implementation costs are the biggest dilemma for the smallest organizations — sized 1-100 employees — a nod to the financial realities and challenges of emerging life science organizations who might understandably have less liquidity in the early stages of their business.
This isn’t groundbreaking information. Scaling companies don’t have access to the same resources and knowledge as enterprise organizations. It is, however, important information, because in our current economic climate and increasingly complex industry, technology risks becoming more and more expensive and unattainable. The long-term dilemma here is that small, albeit potentially important players, aren’t able to claim their seat at the table.
Montrium’s Head of TMF Services, Donatella Ballerini, shares that generally speaking, this finding was the most surprising for her, because “it is not true that the cost of an eTMF is greater than the cost of maintaining a paper TMF system. From archiving, to printing, to purchasing all the tangible resources needed to fulfill a paper TMF, to shipping costs, to man hours and the cost of lost efficiency. If companies took the time to sit down and truly assess the cost of their paper TMF they would see that it’s a false belief that an eTMF is more expensive. This is a false assumption that we need to debunk because an eTMF will save you money in the long run.”
The typical cost of implementing an eTMF system often starts around $15,000, accounting for the technical configuration of the environment, validation, and training. However, this fee can vary based on the eTMF system you select, the size of your organization and other factors.
But for companies with less resources, the sentiment that the cost of implementation is too high only reiterates that the initial barrier to entry for scaling organizations is disadvantageous. For some, even with the comprehension that implementing an eTMF is in fact cost-saving, it’s just not feasible. So — what can e-clinical tech companies do to alleviate this hurdle? We are seeing new pricing models emerge, offering lower and more accessible costing to smaller, scaling life science organizations. This is a start.
Montrium has likewise altered our pricing to ensure accessibility to those companies who see the value in an electronic system but might not have the financial bandwidth for the full system, yet. It’s a question of inclusivity on the part of tech providers operating in such a powerful and consequential space. Holding the key to help sponsors and CROs conduct better, more efficient clinical trials comes with great responsibility.
The buy-in dilemma
In the same vein, 21% of respondents cite “getting management buy in” as a top challenge to implementing an eTMF, highlighting a disconnect between the needs of the clinical operations leaders or end users who are responsible for managing and filing the TMF content, and those who approve the purchase, namely the company’s executive or financial team. This dissonance is an important one to pay attention to as the lack of harmonization around people’s needs does not match the harmonization required within the TMF itself to achieve inspection readiness. With so many moving pieces, moments, documents and narratives to stitch together under a compliant lens, the clinical trial story would benefit from the use of electronic systems that innately promote harmony.
The “now is not a good time” mentality
The second most cited challenge was “time to get up and running” at 22%. Yes, there’s a level of complexity that is typically seen during implementation. Many larger, more complex e-clinical systems require intense configuration and training that takes longer to prepare rollout to users. Plus, the more complex it is, the longer it takes for users to learn it, so there’s an unproductive downtime that needs to be accounted for that likely scares companies off from making the switch.
The truth is, there’s no perfect time to migrate your study into an eTMF or onboard into a new system. It will take time and work efficiently, and it will disrupt your current flow, but that’s not necessarily a bad thing in the long run. Working with the right vendor who has strong migration experience, streamlined implementation processes and an intuitive product will make all the difference.
As smaller sponsors scale, implementing an eTMF will eventually become the golden ticket to a successful expansion. And as the CRO market increases in competitiveness, adopting an eTMF system will become a proud differentiator.
44% of respondents from scaling organizations (1-100 employees) cited cost of implementation as the greatest impediment to implementing an eTMF system.
Implementing an eTMF system: What (do you think) is in it for you?
- 40% – Improved efficiency
- 37% – Enhanced compliance
- 8% – Easier collaboration
- 8% – Better reporting
- 5% – Cost savings
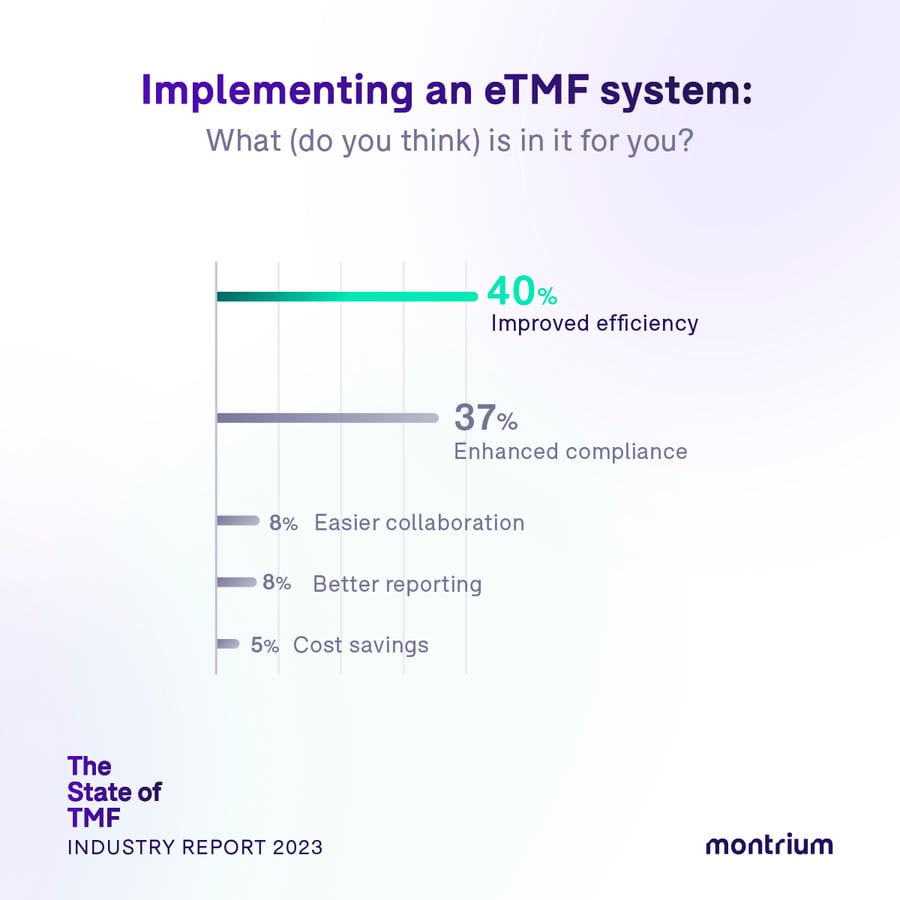
Interestingly, all company size segments cited “improved efficiency” — 40% — and “enhanced compliance” — 37% — as the most significant benefits of implementing an eTMF system. This reveals that an eTMF is considered more of an operational tool, something that brings benefits to the management of clinical trials.
Contrastingly, cost savings only account for 5% of the pie, which leads us to believe that many don’t entirely grasp the actual cost of how they are currently working (within paper or other non-electronic systems) as well as the cost savings that could benefit them when moving to an eTMF. The truth is that improved efficiency = cost savings, so they go hand in hand.
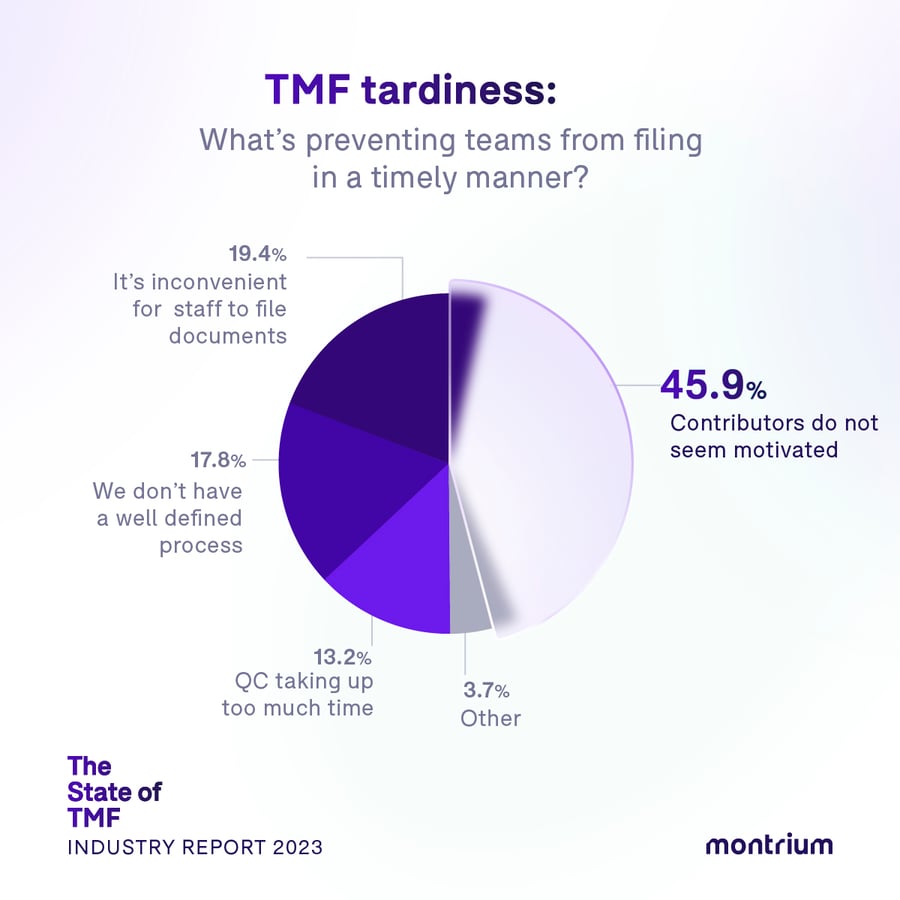
TMF tardiness: What’s preventing teams from filing in a timely manner?
- 45.9% – Contributors do not seem motivated
- 19.4% – It’s inconvenient for staff to file documents
- 17.8% – We don't have a well-defined process
- 13.2% – QC taking up too much time
It’s a people problem
When it comes to getting documents into the TMF in a timely manner, we see that it’s not so much a technological or process problem, it’s a people problem. 45.9% of respondents cited “contributors don’t seem motivated,” as their greatest impediment to timely filing, and this rings true as the biggest roadblock across all the verticals: industry, company size, and type of TMF management.
The greatest limiter of TMF success is people and their sentiments towards the work they are doing, or the environment they are doing the work in.
Finding
45.9% of electronic TMF users cited “contributors don’t seem motivated” as their biggest impediment to timeliness, compared to only 35% of non-eTMF users.
Our takeaway
Simply making the switch to an eTMF will not resolve all TMF-related problems. How you structure and organize the team of TMF contributors, while providing clarity around roles and expectations, has a direct impact on overall TMF performance.
The inconvenient truth: roles and responsibilities are unclear
Oliver Pearce, Montrium’s Director of Growth, shares his thoughts on the matter: “From my experience working with companies on implementing better TMF habits and systems, filing documents into the TMF is often considered a chore. Systems can be clunky and difficult to use, and contributors don’t always consider filing documents to be part of their job. They believe it instead to be an archivist’s or documentation specialist’s job. There’s a detrimental lack of clarity or direction on who is responsible for tasks when it comes to the TMF, and this is what can cause friction and the unfortunate untimely filing of documents, ensuring that inspection readiness remains at an unwanted distance.”
What’s most unfortunate about this finding is the opportunity that’s being missed. Filing in a timely manner offers stakeholders real-time insights and metrics that they can use to make better decisions throughout the course of study. Think of it this way: a sports coach wouldn’t wait for the end of a match to call a time out. You want to take time to analyze what’s not working well as you play the game so you can adjust your strategy as you go to win. In the case of TMFs, to be inspection ready.
Finding
The percentage of respondents who believed that unmotivated contributors are the greatest issue for timeliness, increases as company size increases.
Our takeaway
If roles around the TMF are unclear as a company scales, the details of who is responsible for what in the TMF can fall through the cracks, leaving teams at risk for running into both accountability and motivation issues. On the other hand, larger companies with access to more resources might also decide to implement a dedicated TMF operations team, and this clarity can boost motivation.
What it all boils down to: TMF culture
The trial master file culture within an organization refers to the collective mindset, attitudes, and behaviors of the entire team towards TMF management and compliance. A strong TMF culture fosters a commitment to data integrity, regulatory compliance, and efficient document management. It ensures that all team members recognize the importance of maintaining an inspection ready TMF and are actively engaged in its proper organization and maintenance.
According to Christina Mantzioros, “in most cases, companies have 1-2 people per study — usually clinical trial assistants — who are being sent TMF content from various sources and contributors and they are uploading it vs. the contributors doing it themselves. Roles and responsibilities are often convoluted, without enough emphasis placed on the importance of filing documents in a timely manner. And often those not directly involved in the TMF don’t even know what the TMF is, or that it represents the core of an inspection. So, the crux of the issue is TMF awareness and culture: how people are primed to comprehend the impact of their actions within the TMF, and how each action has the potential to offer a bounty of actionable, real-time insights when the TMF is appreciated and used as more than just a repository”."
Expert tip: How to improve TMF Culture across your organization
Get in a data-driven mindset
Emphasize the importance of the TMF as a source of data rather than a mere repository. As a tool that can be leveraged to show the quality of the conduct of your clinical trial and provide key insights.
Define roles & responsibilities
Who will be responsible for developing or housing TMF content and who will be responsible for uploading those documents to the eTMF? Clarity is everything in your TMF plan.
Provide training on the eTMF system
Designate an eTMF system champion and use them to motivate and train TMF contributors on the features that are relevant to them to be able to contribute to the eTMF.
Conduct continuous monitoring
Check in frequently on TMF activities and the state of the eTMF to look for optimal use or tweak processes to optimize day to day activities and contributor motivation. Collaborate with your eTMF provider for guidance as needed.
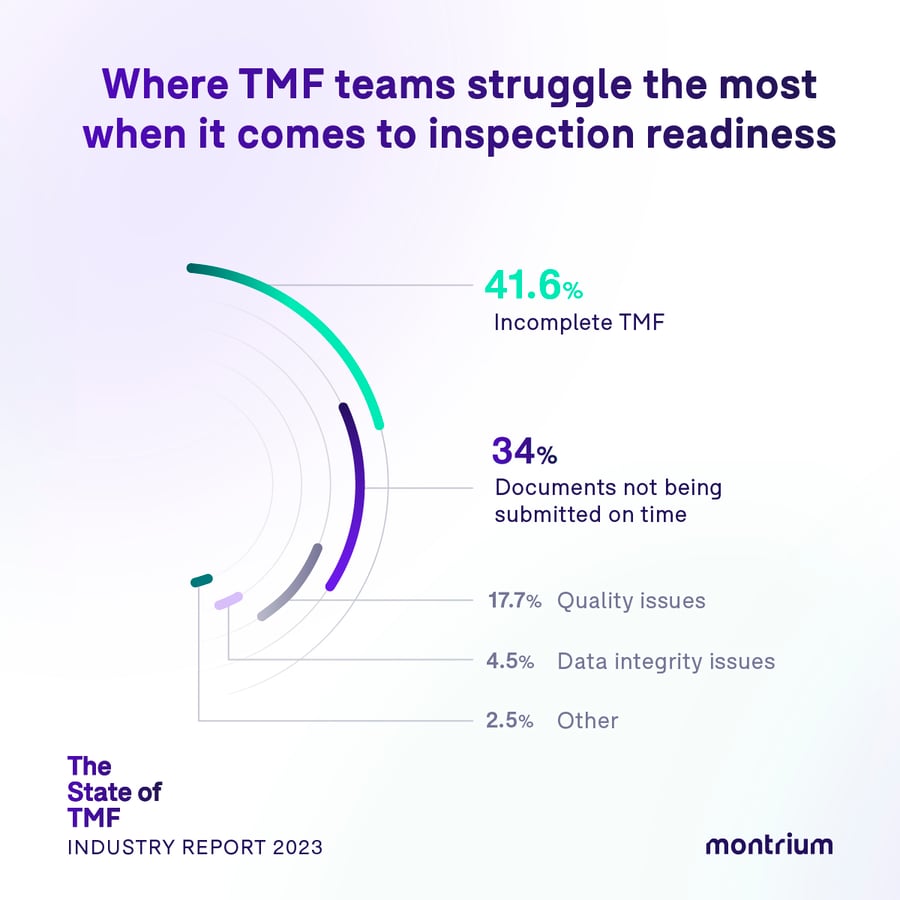
Where TMF teams struggle the most when it comes to inspection readiness
- 41.6% – Incomplete TMF
- 33.7% – Documents not being submitted on time
- 17.7% – Quality issues
- 4.5% – Data integrity issues
- 2.5% – other
Half-baked does not a complete TMF make
We’re not surprised to see that over 40% of respondents cite an “incomplete TMF,” as the greatest impediment to achieving inspection readiness, followed by nearly 34% of “documents not being submitted on time”. The timeliness issue feeds the completeness issue, creating the larger issue of inspection “unreadiness.”
The lack of contributor motivation to file documents in a timely manner – if at all – can quickly become a liability for studies. What emerging organizations need are engaged TMF contributors who understand the monolithic importance of their role of ensuring an accurate re-telling of the clinical trial story.
The completeness “mirage”
But, the conundrum of the incomplete TMF is a larger, ongoing problem. And even when you might think it’s complete, it’s more likely what we like to refer to as the completeness "mirage.”
Why is that?
Most systems today don’t offer reliable metrics. They’re based on a TMF index designed at the beginning of the study. Completeness, however, should be based on study events and other processes. Ultimately, organizations need to be able to define what completeness, quality and timeliness mean in their organization, as well as the parameters used to monitor them.
For example, a document (such as a PI's CV) may exist somewhere (such as the site's investigator file) but it’s not yet in the eTMF. So, what are the parameters here that define the timeliness for this document? Is it comparing the date of the PI's signature on the CV to the eventual date of upload to the eTMF? Should this be less than 30 days, 60 days, etc? Ultimately, eTMF systems should offer this type of configurability of parameters so that metrics are readily available and auto calculated.
Beyond measure: The most difficult TMF metrics to track
- Completeness – 41%
- Timeliness – 23%
- Compliance with procedures – 21%
- Quality – 15%
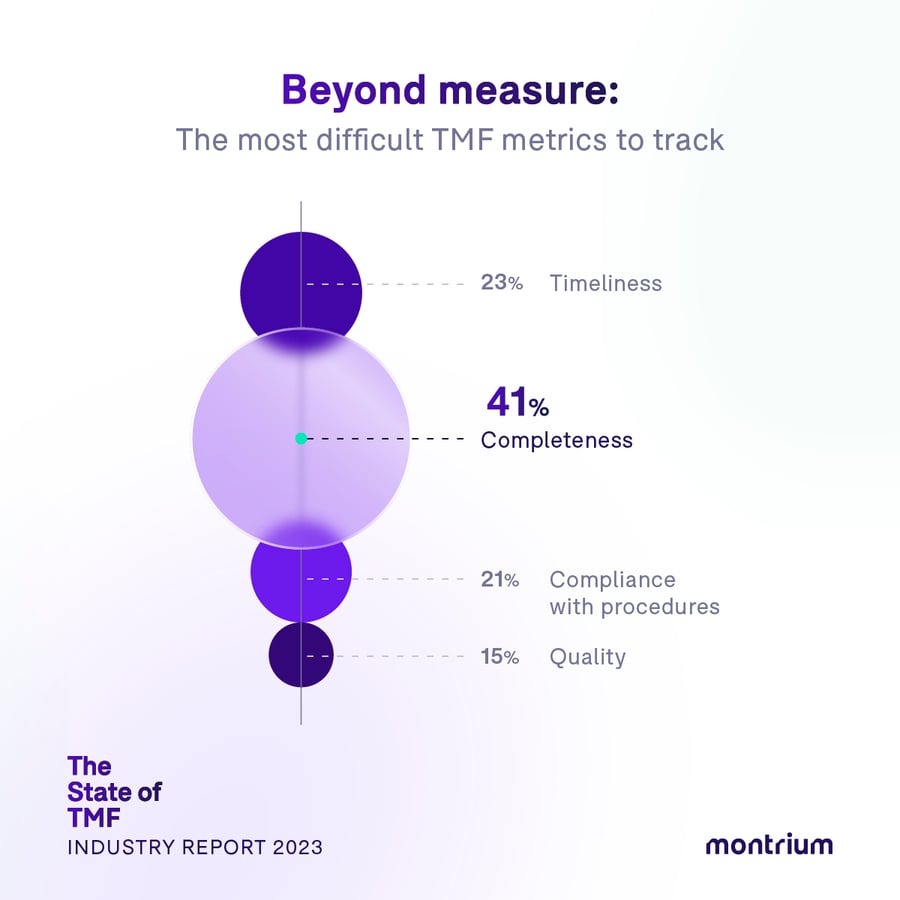
When it comes to measuring TMF metrics, completeness seems to be the one giving TMF professionals an exceptionally hard time. 41% of all respondents remarked that completeness was the most difficult metric for them to track and monitor. In comparison, the second most difficult metric—timeliness—rang in at only 23%. What might be at play here?
Parts of a whole: Stakeholder-driven completeness
Completeness has long been a complicated metric to measure accurately. While there may be a tendency to attribute these difficulties to ineffective tools and processes (e.g. not utilizing eTMF placeholders to their fullest extent), our Head of eTMF Services, Donatella Ballerini, has a different hypothesis: people and culture. “The main reason that people struggle with completeness is the lack of involvement of all relevant TMF stakeholders. People routinely think of the TMF as strictly a clinical development affair, yet that’s not necessarily the case. Many other stakeholders also produce documentation for the TMF, whether it be sites, vendors, laboratories, pharmacovigilance—there are a lot of different parties producing documents. However, these stakeholders are not all aligned on the expectations for the trial master file; indeed, some of them don’t even know it exists. So, if the expectations, management, and processes aren’t established with all stakeholders from the beginning, your TMF will never be complete.”
Quality qualms: What’s slowing down QC?
- 26.6% – Not enough staff
- 26.6% – QCing 100% of documents
- 22.4% – QC rushed due to untimely filing
- 21.6 % – Frequent document quality issues
- 2.9% – Other
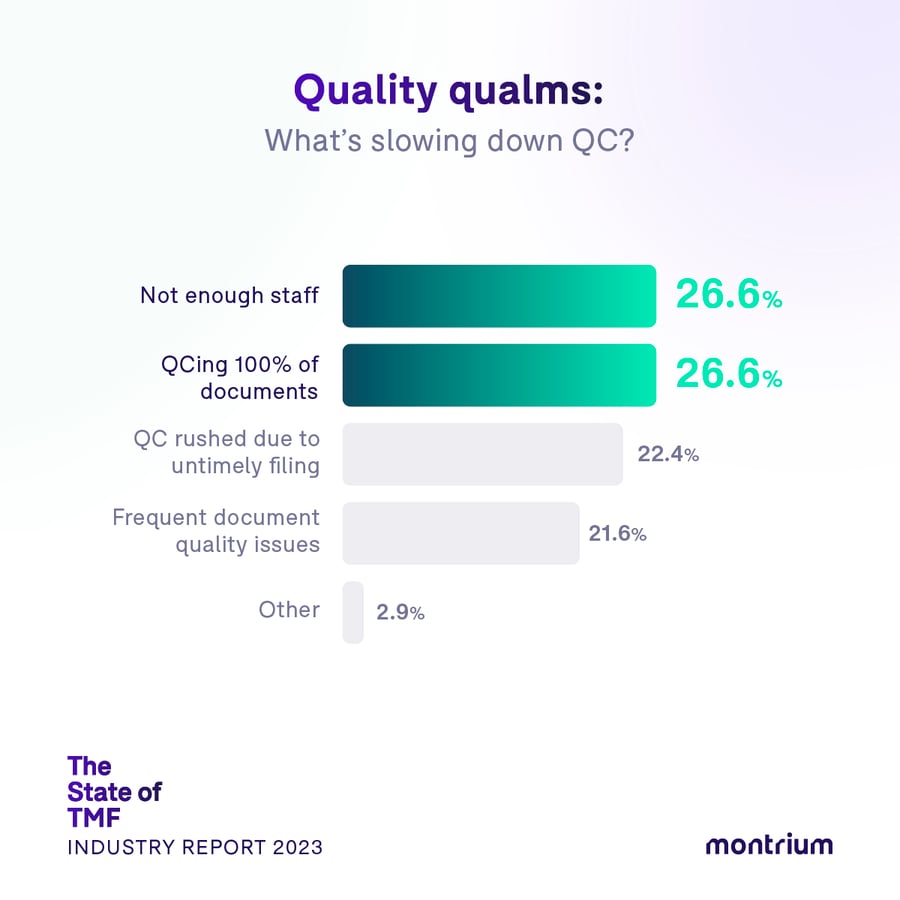
The two most-cited impediments to efficient QC at respondents’ organizations were that they didn’t have enough staff and that they were performing QC on 100% of documents, as opposed to leveraging risk-based QC. While risk-based QC has steadily grown in popularity amongst life science organizations and regulators, the results of our survey indicate that it has yet to be adopted across the board and its full potential has still not been realized. Of those organizations performing QC on 100% of documents, it seemed that CROs were significantly affected by this problem, with nearly 1/3 citing this as their greatest impediment.
Growing pains: Struggling to build a QC dream team
Turning to those who answered that not having enough staff was the biggest impediment to efficient QC, an interesting trend emerged. Whilst not having enough staff was the most-cited impediment amongst small companies (i.e. those with 1-100 employees), it was also the most-cited impediment amongst mid-size companies (with 500-1000 employees), and the second most-cited amongst larger companies (over 1000 employees). Indeed, personnel constraints would appear to be a logical impediment for smaller companies, but for larger organizations, it’s less evident why this would be a hindrance. Is it due to ineffective scaling? Or just employees’ individual perceptions of their companies?
All in all, however, the distribution of responses to this question was rather equal—and Christina Mantzioros, has a theory as to why. “I think this illustrates that QC is a point of contention in the TMF world. Organizations lack a proper plan for QC scope, conduct, and responsibilities, in addition to not leveraging the tools that should make it easier to conduct and document QC. While it may be tempting to ascribe inefficiency to personnel shortage, there are likely larger factors at play given how important the TMF is.”
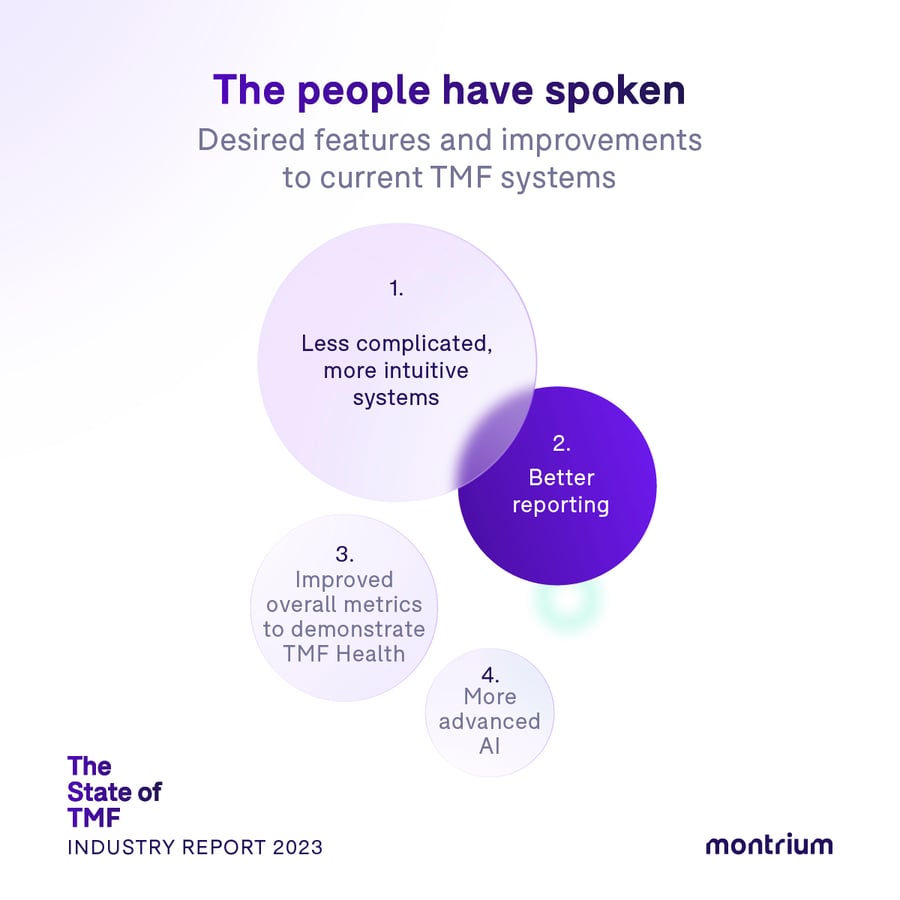
The people have spoken: Desired features and improvements to current TMF systems
In this final survey question, we uncover our community’s “wish list” of features and improvements they’d like to see in today’s TMF systems. While teams across the board have their unique sets of challenges and needs, we are able to surface a set of recurring themes in the data.
These aren't new topics. We've been talking about AI in TMF for nearly five years and we're seeing a much greater willingness in heavily regulated spaces to explore what it can do to streamline processes. Expect more to come here. The same goes for more simplified tech. As younger teams enter the clinical workforce, tech must become more enabling, reflecting the apps and technology we use in other spaces. To motivate people around eTMF tech, we believe that the current interface of many systems needs to be modernized. This is top of mind for Montrium.
1. Less complicated, more intuitive systems
2. Better reporting
3. Improved overall metrics to demonstrate TMF Health
4. More advanced AI
Let’s continue the discussion
While we would like to acknowledge once more that the opinions expressed in this survey do not reflect the thoughts of every TMF professional, we nevertheless find ourselves extremely pleased with the quality of the trends we’ve uncovered. With the information synthesized from this dataset, we’ve been able to shed light upon the understandings and sentiments that are shaping the industry as we know it. We’ve touched upon a variety of different—yet interlinked—aspects that include everything from inspection readiness, to metrics, to electronic systems, and beyond. And this is only the beginning. We will continue to conduct, analyze and share original research well into the future, so we invite you to keep your eyes peeled for more surveys from our team.
Thank you for taking the time to read the State of TMF Industry Report 2023. We invite you to share your thoughts, opinions, and questions about the report on LinkedIn using the hashtag #StateofTMFReport2023, or join in on the discussion about this report on the Montrium LinkedIn page alongside our engaged and thoughtful community of followers.
Grab your own copy of The State of TMF Industry Report 2023
We've created a .pdf version of this report that you can download and share with others. Plus, you'll be able to see the full appendix of the data we collected during the survey.








-1.png)

.png)
-1.png)


.png)
-3.png)
