-1.png)
History is full of tales about how technological and process innovations have shaped society for the better. Take the printing press, for example. Something major shifted when goldsmith Johannes Gutenberg invented the moving-type printing press circa 1440. It represented the dawn of a new era in which information and ideas could be documented en masse and distributed to people far and wide.
And, if we think to the present day, technology has evolved to such an extent that we no longer even require tangible, paper-and-ink documents to communicate with one another—something that would’ve surely knocked Gutenberg’s socks off.
But what does this have to do with the trial master file? Bear with me.
Let's dive in!
Let’s Face It: TMFs Are Stuck in the Past
The TMF industry has come a long way since the ICH GCP E6 Consolidated Guideline in 1996 first mandated the need to maintain a trial master file. We’ve collectively witnessed clinical trial providers go from filing cabinets to eTMF systems, from express mail to email. Yet, it seems that the culture of our industry hasn’t quite caught up to the technology we now possess. We continue to see organizations accumulating inspection findings over issues that could’ve been easily avoided by making use of the state-of-the-art systems at their fingertips, and we still bear witness to an unsettlingly large share of companies treating the TMF as a repository.

What gives? Do we all just really want to keep doing things the hard way? Would life science organizations have fought to continue writing documents by hand while the printing press was revolutionizing and expediting that very same process? I doubt it—and I hope you do, too. The prevailing culture around TMF management has imposed a massive roadblock on our path forward as an industry. To overcome it, we need to change our mindset, our strategy, and our processes to match the technology available to us and the best practices we know to be true. We need to begin practicing active TMF management.
The Challenges of Passive TMF Management
Passive TMF management has been the de facto mode of operation for many life science organizations for what seems like forever. A bygone from the days of paper TMFs, it’s characterized by “filing cabinet syndrome,” the view that the TMF is little more than a repository or a box to be checked off for regulators. In short, it’s a nightmare for compliance. But what is compliance, really? Well, let’s think about it in the context of timeliness, completeness, and quality—and the effects that passive TMF management has on all three.
When it comes to timeliness, passive TMF management fails to offer any incentives for filing on time. Documents are filed at the end of the study—or right before an inspection—and then rarely accessed again, meaning that the task of filing perpetually occupies a spot at the bottom of the to-do list. As a result, it becomes difficult to get the necessary content into the TMF, making oversight nearly impossible to perform and rendering completeness calculations all but moot. With documents not being submitted in a timely manner, it becomes exceedingly hard to resolve potential issues as time passes—not to mention the additional complexities thrown into the mix by staff turnover. All of this portends massive issues with respect to quality and, of course, inspection findings.
Passive TMF Management Is More Than Just Inefficient: It’s Risky
In the current regulatory environment, this is a risky approach—to put it lightly. With the adoption of Regulation (EU) 536/2014, it’s now a requirement that the trial master file “shall at all times contain the essential documents relating to that clinical trial which allow verification of the conduct of a clinical trial and the quality of the data generated.” Basically, this means that the TMF can no longer be regarded as an afterthought and haphazardly compiled at the end of the study. It needs to be updated regularly and contemporaneously with each development.
And, with the widespread adoption of eTMF systems and the visibility they provide, it’s no longer possible for untimely filing practices to hide in the refuge of paper documentation. Audit trails, reporting, and other such features have spelled the end of passive TMF management. Inspectors are now able to identify this type of TMF management with ease—and dole out findings accordingly.
Active TMF Management: What Does It Look Like?
By now, the woes of passive TMF management are rather self-evident, and so too is the antidote: active TMF management. But what does active TMF management actually look like? Well, it’s pretty much exactly what it sounds like. It involves actively contributing to and accessing the TMF as part of your overall clinical trial process. In this way, the TMF is brought to the forefront of clinical trial management instead of being relegated to a loose end that must be tied upon study close-out.
Paradoxically, ongoing maintenance and upkeep of the TMF actually saves you time once you embrace the concept and harness the project management power of your eTMF system. How, you ask? For starters, historically labour-intensive processes such as content collaboration—that is, the authoring, review, and approval of documents—can be carried out directly in the eTMF system itself. Gone are the days of sending numerous drafts back and forth to one another, or having to purposefully create and file evidence of the document approval process.
The Makeover Your TMF Desperately Needs
Streamlined content collaboration is great, of course, but the true benefits of active TMF management materialize when you take a step back and consider the TMF as a whole. Imagine, if you will, an alternate universe in which you have holistic, comprehensive oversight into everything that’s going on within your TMF.
You can accurately gauge completeness, you have real-time insight into your TMF health, and quality issues are a thing of the past. With active TMF management, that alternate universe can become a reality. How?
- Predictive completeness allows you to proactively leverage placeholders and signposts in order to track the progress of your trial and gain a true understanding of TMF completeness.
- An integrated eTMF plan means that you always know where everything is supposed to go, empowering you to get a handle on TMF health.
- Risk-based QC helps to ensure that quality problems are diagnosed and resolved efficiently, helping to save time by focusing on the areas that present the highest risk.
In addition to returning valuable hours to your workday and improving the performance of your TMF all around, active TMF management also helps to instill inspection confidence. When you already know where everything is and what state it’s in, you can sleep soundly at night knowing that there won’t be any surprises during the course of an inspection. And inspectors can tell quite easily whether or not you’ve been practicing active TMF management. They know just as well as you do that if everything has been filed at the last minute, then there probably exist myriad quality issues permeating your TMF.
So, think of active TMF management as a process in which you spend a little bit of time now to save yourself a lot of time in the future—and save yourself from inspection findings.
How Can We Shift to Active TMF Management?
I’ve now spent a considerable number of words touting the benefits of active TMF management and highlighting the consequences of not practicing it—which is great, because it’s exactly what I set out to do. However, I still have yet to answer the question of how. How can you make the shift to active TMF management? This is where things get interesting. To successfully transition from passive to active TMF management, you’ll need a three-pronged implementation strategy—a fork, if you will. Each prong is related to a specific building block of your organization: people, processes, and technology.
-1.png?width=1200&height=627&name=MicrosoftTeams-image%20(27)-1.png)
People
It’s all too easy to focus only on processes or technology when thinking about TMF management. However, in not broadening your scope to include people, you would fail to take into account the true vectors of change within an organization. After all, people are the ones who develop and abide by the processes, who invest in and use the technology, and who, at the end of the day, are the ones creating and filing the documents within the TMF. Thus, it’s important that change is not only embraced by the people within your organization, but that it actually originates from them as well.
Indeed, upskilling and training employees is crucial when shifting to a new model of TMF management. But so too is involving them in designing an active TMF management process that moves away from the archivist mindset and orients the roles of TMF professionals around more strategic, data-centric initiatives. By taking a grassroots, bottom-up approach and soliciting input from end users, you not only guarantee that your new process covers all the bases, but also that employees have a vested interest in its success.
Process
Your process is the engine that drives your clinical operations. You need to ensure that it’s robust, powerful, and capable of getting you where you need to go. When designing a new process to support active TMF management, you need to begin with your end goal in mind. What are your objectives for completeness, quality, and timeliness? Overall efficiency and compliance? Once you know what you want to achieve, you can work backward by breaking the journey down into more granular, concrete, and measurable objectives that feed into your overarching goals.
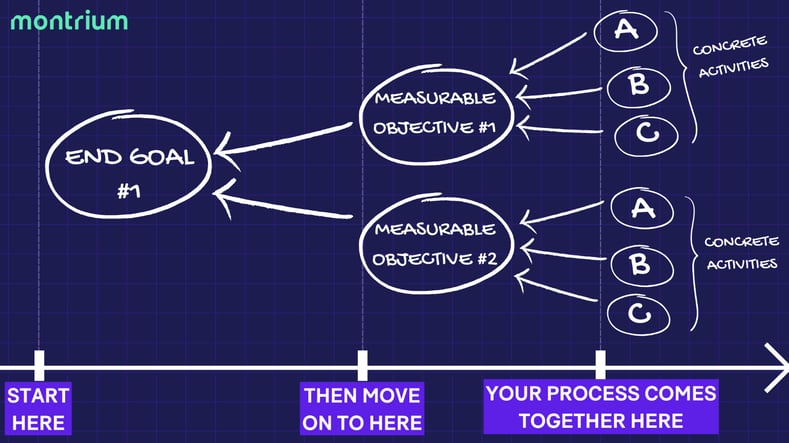
You will, of course, need to place an emphasis on regularity and proactivity. This means provisioning for frequent document reviews, shifting collaborative processes to your eTMF system, and creating frameworks for data-based monitoring. This may be uncomfortable at first; roles and responsibilities may shift, new skills may need to be learned. But by keeping your end goal in mind, the value of your new process will become readily apparent.
Technology
Lastly, you need to ensure that you have a system in place that not only enables, but empowers active TMF management. To be frank, it’s simply not possible to achieve all of these things in a paper—or even hybrid—environment. Your eTMF system needs to become your active TMF management HQ, a place where you’re able to collaborate and iterate, oversee and analyze. You need to ensure that you have a system capable of supporting programs like risk-based QC, predictive completeness, and TMF health monitoring. Your system should prioritize ease-of-use and reduce friction in your processes in order to remove barriers to active contribution. When all is said and done, your eTMF system should serve as a nexus through which people and process work in harmony to execute effective TMF management.
Final Thoughts on Active TMF Management
It’s all too easy to ascribe the persistence of passive TMF management to laziness, but doing so would ignore some fundamental truths about our industry—namely that, as a whole, life sciences professionals are hardworking and dedicated to pushing the boundaries of innovation. Instead, we need to look at the root cause of ineffective TMF management practices for what it truly is: a matter of culture. By consciously rethinking our approach to the trial master file and embracing the tenets of active TMF management, we can propel the clinical research industry forward to the benefit of professionals and patients alike.







-1.png)





.png)
-3.png)