Trial master files represent a significant challenge in today’s clinical trial environment due to the sheer volume of documentation, the widely distributed geographic location of clinical sites and the global nature of many studies.
Traditionally, TMF managers have constructed complex excel trackers to monitor TMF completeness and ensure that documentation is collected and completed contemporaneously. While this does give TMF managers some oversight on how healthy their TMF is, it still requires a significant amount of manual effort in maintaining the trackers – not to mention the increased risk of errors if any information is mismanaged (we’re all human after all).
Many eTMF software vendors have now begun the journey into providing greater insights into TMF health by adding business intelligence dashboards on top of their content management tools. However, while vendors may claim to provide great TMF insights, it’s important to understand what is truly required to measure and monitor TMF completeness. We can generate all of the graphs, tables, and reports we can possibly muster, but if they aren’t actionable and don’t drive performance, they’re actually not delivering additional value.
In the following article, we’ll touch on what eTMF completeness really means, how to measure it and what key features you should be looking for as you search for solutions with TMF completeness reporting.
What is eTMF Completeness?
The most important measurement in managing trial master files is TMF completeness. Being able to provide all of the documentation collected over the course of a clinical trial in an organized and auditable way that ensures that the trial can be reconstructed and that we demonstrate proper controls were in place to protect patient safety is the ultimate goal. These controls are primarily found in our ability to set placeholders for the TMF artifacts that we’re required to collect for the trial.
The advantage of having a tool that generates placeholders for expected artifacts is in our ability to understand the quality and health of our TMF. We use these placeholders as a tool to understand how complete our TMF is – and in turn report on that completeness as we prepare for audits or inspections.
The Challenges of eTMF Completeness Calculation
While eTMF completeness is the obvious end goal, it’s important to understand that eTMF completeness is not an exact science, and there are significant challenges with implementing an accurate completeness program. As you plan to transition to a more intelligent eTMF completeness program, be wary of the following challenges.
- Accuracy is a big issue
- Much content is unstructured i.e. emails
- Dependent on study design, regional requirements, study events, study organization and milestones
- Systems need to be more agile in being able to calculate completeness intelligently based on these attributes
- Connectivity between systems and sharing of information is the foundation of being able to calculate completeness accurately
- The TMF RM exchange standard may be able to help with this in the future
How to measure eTMF Completeness?
At each stage of our study, there are milestones that demonstrate the progress we’ve made. Each of these milestones requires a minimum set of documentation that is expected to be collected at that stage. For example, if one of our sites has a status of identified, we are expected to collect a finalized site confidentiality agreement and in some case a debarment certification statement.
By setting these stages and managing expected documents we can measure how complete our TMF is at any point in time, and work to obtain required documents from the site without delay. Most eTMF software tools on the market should provide you with a set of static reports for understanding eTMF completeness, based on what’s expected at a specific stage. However, more and more we are hearing from our customers at Montrium that they require more creative tools to track completeness.
In the next section, we’ll explore some of the key features that you should expect in an eTMF completeness report.
Key Features of an eTMF Completeness Report / Dashboard
Consistent Hierarchical Study Structure
At a minimum, your eTMF completeness dashboard/report should enable you to formulate a logical hierarchical study structure. Whether you’re aligning to an existing model (like the TMF Reference Model structure), or mapping to your own internal structure, the report/dashboard should have the ability to display and manage TMF content in this structure.
Dynamic Nature of Completeness
Completeness is an ongoing activity, and thus your TMF completeness tools should reflect the dynamic nature of studies. As you add sites or countries, you’ll want to understand what’s required at each stage, and what is missing from previous milestones. Our own eTMF Navigator has this ability, as well as the capacity to track study milestones across your study.
Performance & Scalability
Most clinical studies require hundreds of thousands of documents to be collected from many sources and stakeholders. Your TMF completeness tools will need to be able to handle the volume you expect, and for the tools to scale as your trial scales. Static reporting will provide you with something that is just that; static. Whereas a dynamic reporting interface can be modified and changed as you make decisions. It goes without saying that your tools should accommodate this need.
Intuitiveness & Clear User Interface
eClinical systems, in general, have traditionally been large complex repositories where an immense amount of navigation is required to find the content you need and complete actions. As previously mentioned, managing a TMF can mean dealing with large volumes of documentation, so being able to navigate the web of information in an easy to use interface is critical.
Filtering and Drilling Down
Trial Master File content has a world of information associated with each artifact, which in the right tool can mean a tremendous about of additional data to trend on. Having TMF completeness tools that allow you to filter and drill down even further gives a multi-dimensional view of your trial master file. This is especially valuable as you begin to prepare for audits or inspections, isolating expected or missing documents and following up with the responsible individuals.
Logical & Meaningful Statuses
While this may seem an obvious feature, too often, we see eClinical systems packed with difficult to understand jargon that confuses its users. It’s important that as you set up your TMF completeness program within your organization that there are logical and meaningful statuses in place. Additionally, look for tools that come pre-configured with statuses that make sense – it will save you time and effort of having to re-configure something that doesn’t represent how you manage your studies.
Security & Data Protection
Restricting access to blinded documents is an integral part of many clinical trials, however, protecting blinded documents in electronic systems is not always straightforward nor obvious. Your eTMF completeness report must have the ability to provide completeness metrics and information on the study, without revealing specific information that could break the blind.
Document Previewing
Throughout the course of your clinical trial, you’ll be receiving copious amounts of documentation to file in your TMF. With a multitude of content being received by your team, having the ability to preview documents as you import them into the system will help you index and associate the relevant metadata faster. While not technically an essential part of an eTMF completeness report, being able to add content to your TMF through your completeness report significantly improves the time it takes to reconcile TMF content and brings TMF completeness to life.
The Impact eTMF Completeness Reporting has on your Clinical Study
The ability to get real-time business intelligence on the health of the trial master file, not only allows organizations to make better clinical decisions but helps them prepare more efficiently for audits and/or regulatory inspections. If you can save an hour a day by using an intuitive tool that will not only have a significant financial impact but will most certainly give you additional time to be more involved in your clinical programs.
Recommended Approach to eTMF Completeness
- Start with the easiest attribute – milestones
- Build eTMF plans based on specific study designs
- Identify study events which would require artifacts e. protocol amendment, safety case
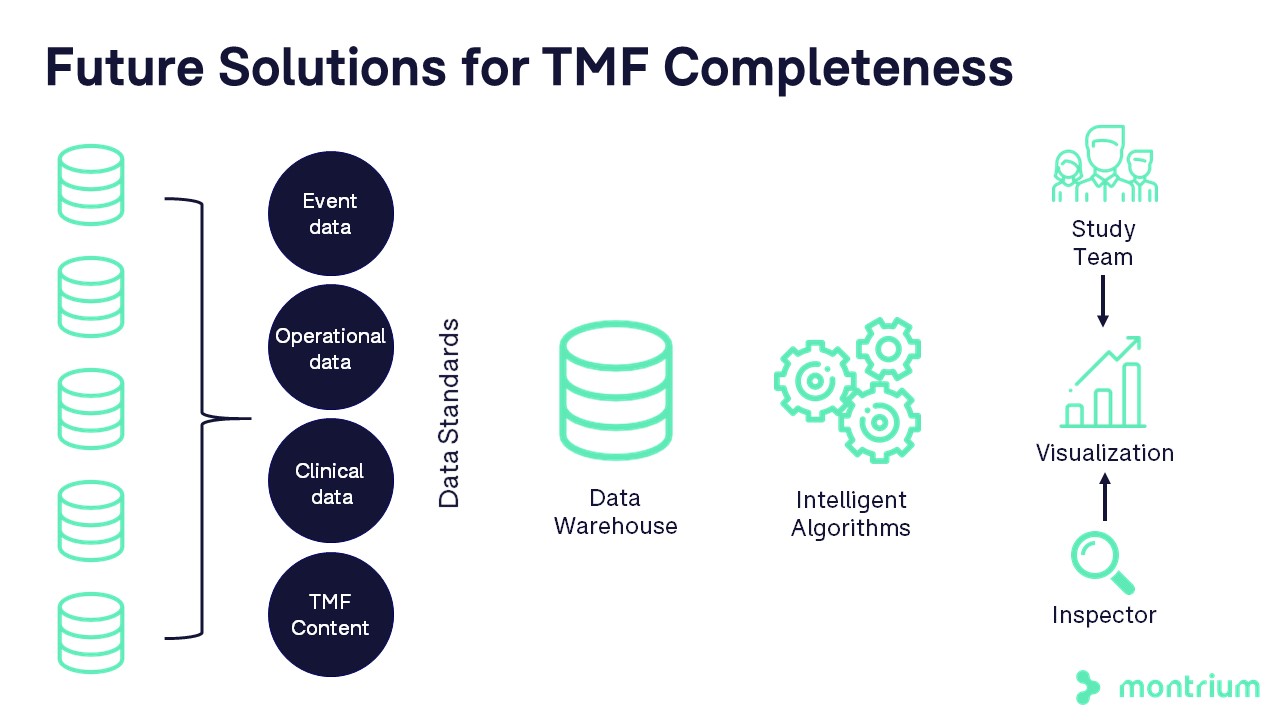
- Consider systems integration and/or data warehouse to improve the accuracy of event data
- Evaluate data needs to identify and calculate completeness of artifacts contained outside of the primary system
- Implement an eTMF completeness program to build up capabilities over time
- Leverage historical data and predictive algorithms to improve accuracy