Last updated: March 4, 2026
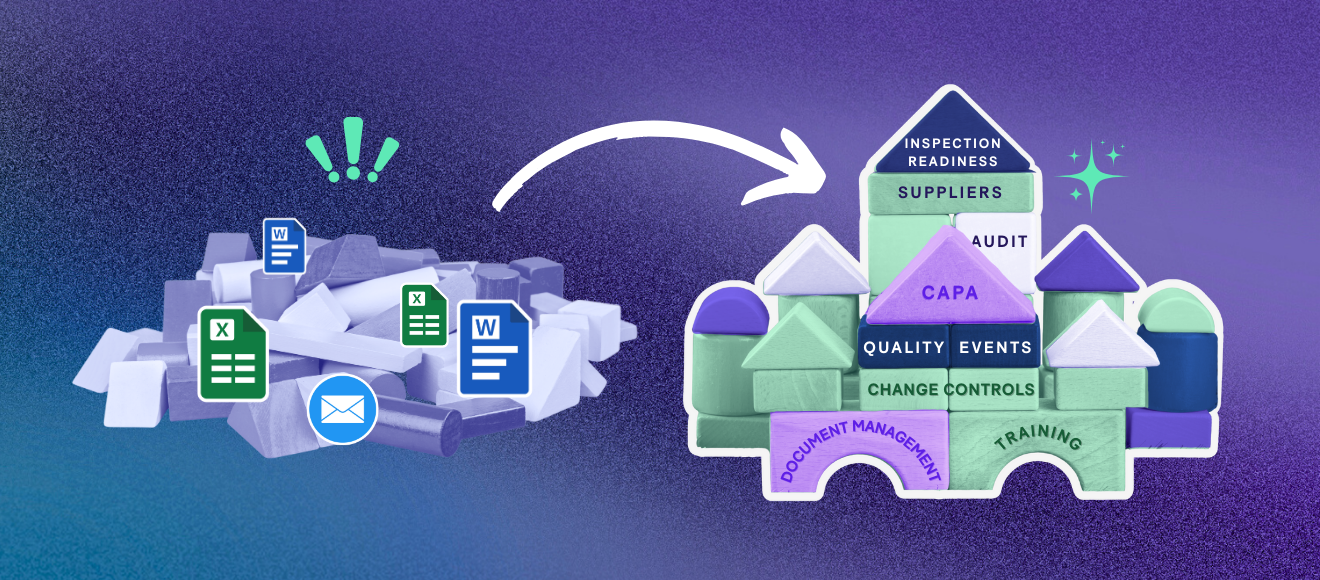
What is an eQMS?An eQMS (electronic Quality Management System) is a cloud-based software platform that centralizes and automates quality management processes for life sciences organizations.
It replaces paper-based and spreadsheet-driven systems for document control, training management, quality event tracking, and CAPA management. All quality activities, approvals, and records are stored in one validated system with full audit trails.
For regulated organizations, an eQMS is built to meet FDA 21 CFR Part 11, EudraLex Annex 11, and GxP (Good Practice regulations) requirements, with electronic signatures, configurable workflows, and system-generated compliance reports.
What are the benefits of an eQMS software?
We've covered the full breakdown in our dedicated article on eQMS benefits, but here are the most common improvements organizations see after implementation.
Scale without adding headcount
Your QA team manages a growing organization without proportional staff increases. One person can handle training and SOP management for a 100+ person team.
Onboard new employees faster
New hires get assigned the right training automatically based on their role. No manual tracking of who needs what before they start work.
Catch compliance gaps before regulators do
Real-time reports show overdue trainings and missing approvals before an inspection is scheduled, not after.
Stay compliant as regulations change
A purpose-built GxP system keeps your quality processes aligned with 21 CFR Part 11, Annex 11, and ISO standards without rebuilding your workflows every time requirements shift.
Reduce risk from version control errors
Everyone works from the current approved version. Outdated SOPs don't circulate, and you have a full record of every revision.
Speed up study startup
Quality documentation and training are in order before a new study begins, so compliance gaps don't delay your clinical programs.
What modules are typically part of an eQMS?
Not all eQMS platforms are built the same. As you evaluate systems, look for one that covers your core quality processes today and scales with your organization as you grow. Here are the modules you should expect to see.
Document control
Your document control module manages the full lifecycle of SOPs, policies, and work instructions. It stores, organizes, and retrieves quality documentation, routes documents for review and approval through automated workflows, and maintains version control so your team always works from the current approved version. Look for native Microsoft Word integration so authors work in familiar tools without switching between systems, like Montrium’s Quality Connect.
Training management
When a new SOP is approved, your team needs to train on it. Your training module should connect directly to document control, automatically assigning training to the right employees based on their role when a document is released. Real-time reports show who is compliant, who is overdue, and where gaps exist before an auditor asks.
Quality event management
Deviations, complaints, and non-conformances need to be captured, tracked, and closed in one system. A quality event module replaces Excel trackers and email threads with structured forms, automated routing, and a clear audit trail from event capture to closure.
Corrective & Preventive Actions (CAPAs)
CAPAs address the root cause of recurring quality issues and put controls in place to prevent recurrence. Your CAPA module should support root cause analysis, action assignment, due date tracking, and closure verification, all with a documented record for inspections.
Change control management
Life sciences organizations are required to manage changes to products, processes, and systems in a controlled, documented way. A change control module structures the full change process from initiation through impact assessment, approval, implementation, and review, ensuring no undocumented changes slip through.
Audit & supplier management
These two modules belong together. Regulations require you to qualify and monitor the vendors and suppliers you work with. Your eQMS should let you store supplier documentation, conduct internal and supplier audits, assign findings, and track corrective actions through closure in one place.
Essential features of your eQMS
The modules in your eQMS are only as useful as the features that connect them. When quality data stays siloed, you lose visibility across your organization and compliance gaps go undetected. The features below form the foundation of any eQMS worth evaluating.
Cloud-based platform
A cloud-based eQMS reduces implementation time and total cost of ownership. Your team accesses the system from any location, and you are not responsible for maintaining on-premise infrastructure or managing system updates.
Web applications
Web-based access means every user, whether at headquarters, a remote site, or working from home, works in the same system with the same real-time data.
Streamlined workflows
Automated workflows route documents, training assignments, and quality events to the right people at the right time. Your team stops managing processes manually and starts working within a system that drives compliance forward.
Electronic signature
Your eQMS needs 21 CFR Part 11 compliant electronic signatures so approvals happen wherever your team is located. No printing, scanning, or chasing wet signatures.
Electronic forms
Structured forms capture quality event data consistently across your organization. Intelligent forms adapt based on the type of event being reported, so your team captures the right information every time.
Structured quality data
Comprehensive metadata management lets you drill into quality data, generate meaningful metrics, and identify trends. You need this to demonstrate compliance and make informed decisions about where quality issues are concentrated.
PDF rendering
Automated PDF rendering converts approved documents and completed forms into long-term archives. Your quality records are preserved in a format auditors expect, without manual file conversion.
Automated training management
When a procedural document is updated or revised, training assignments go out automatically to the employees who need to complete them. You define training requirements once per document type, and the system handles the rest.
Built-in regulatory compliance
Your eQMS should include documented evidence of testing in line with 21 CFR Part 11, Annex 11, and GxP requirements. This is not a feature to verify after purchase. Confirm it before you sign.

When do you need an eQMS?
You need an eQMS when paper-based processes, distributed teams, or an approaching inspection expose compliance gaps your current system can't close.
Most organizations reach this point from one of two directions: implementing an eQMS for the first time, or replacing a legacy or on-premises system that no longer keeps up.
Paper is holding your team back
Paper-based systems create real problems for life sciences teams, especially those spread across multiple sites or countries. Documents get lost, versions go out of date, and collaboration slows to a crawl. The time your team spends managing paper, filing physical documents, chasing signatures, and maintaining binders, is time not spent on quality work.
For geographically distributed teams, paper is not a compliance strategy. It is a liability.
You are not truly inspection-ready
Maintaining inspection readiness is not a one-time event. It requires real-time visibility into training compliance, SOP status, and open quality events at all times. Without a system that surfaces compliance gaps automatically, you only find out you are not ready when a regulator shows up. By then, the cost of the gap is much higher than the cost of fixing it would have been.
Research suggests that only about 10% of organizations using an eQMS have fully integrated it across their quality processes. Most teams are still managing quality in fragments, with disconnected systems that limit visibility and slow down compliance work.
A system that connects document control, training, and quality event management in one place is what closes that gap.
Regulatory requirements keep changing
Regulatory guidance evolves. For organizations managing quality on paper or in spreadsheets, every update requires manual reviews, document revisions, and re-training across your team.
An eQMS built for GxP environments embeds compliance requirements into the system itself. When requirements shift, your workflows and controls shift with them. Your team stays compliant without rebuilding processes from scratch every time guidance changes.
What is the best eQMS for a small biotech or pharma company?
Not all eQMS platforms are built for the same environment. As you evaluate your options, the differences between platform types matter more than the feature lists suggest. Here is what you need to know.
Generic and legacy eQMS platforms
Generic platforms cover the basics of quality management but are not built for any specific industry. For life sciences organizations, this usually means additional configuration, customization, and validation work just to meet your regulatory requirements.
You end up paying more in implementation time and internal resources to make a general-purpose system fit a regulated environment it was not designed for.
Some of the most widely adopted platforms in this category are extremely feature-rich. But feature-rich does not always mean the right fit. These platforms tend to be structured and rigid by design, which works well for large organizations with dedicated IT and validation teams to configure and maintain them.
For a scaling biotech or pharma company without that internal bandwidth, you often pay enterprise-level costs for a system you need to heavily customize before it works the way your team actually operates.
If your organization is in an early or mid-growth phase, the implementation timeline, cost, and configuration burden of these platforms often outweighs the benefits. A system purpose-built for life sciences quality processes gets you to compliance faster, with less overhead.
Medical device eQMS platforms
Medical device platforms are purpose-built for medtech organizations and include modules for design control and product lifecycle management. If your organization is not in medical device development, these systems carry complexity you do not need. Even for medical device organizations with straightforward quality needs, the overhead of a full medtech platform is often more than the situation calls for.
eQMS built for life sciences
A life sciences eQMS is built around the quality processes your team already follows. Document control, training management, CAPA, change control, and audit management are all designed for GxP environments from the start. You get 21 CFR Part 11 and Annex 11 compliance built in, without the configuration burden of a generic system or the complexity of a medtech platform.
For biotech and pharma organizations scaling through clinical development, this is the category worth focusing your evaluation on.
How much do eQMS systems cost?
Cost is one of the first questions that comes up when building an internal case for an eQMS. The honest answer is that it depends on the type of system, the size of your organization, and the complexity of your quality processes. Here is what to expect.
Implementation fees
Every eQMS worth evaluating includes some form of implementation fee. This covers configuration, onboarding, and initial training to get your team up and running. The cost varies based on organization size, the number of quality documents to migrate, and how complex your workflows are. Larger organizations with more complex rollouts will pay more. Ask vendors to break down exactly what the implementation fee covers before signing.
Data or document migration
Most organizations migrate at least some of their existing quality records into their new system for continuity. Migration cost is driven by volume and complexity. The more content you are moving, and the more complex your existing records are, the higher the cost. Ask vendors upfront whether migration is included in the implementation fee or invoiced separately.
Employee training
Leading eQMS providers include training in their implementation fees. Some vendors split it out to lower the headline cost, so confirm what is included before you sign. If a vendor offers only self-serve, on-demand training, consider whether your team will get enough support from that model or whether gaps in training quality will create problems down the line.
User licenses and your subscription
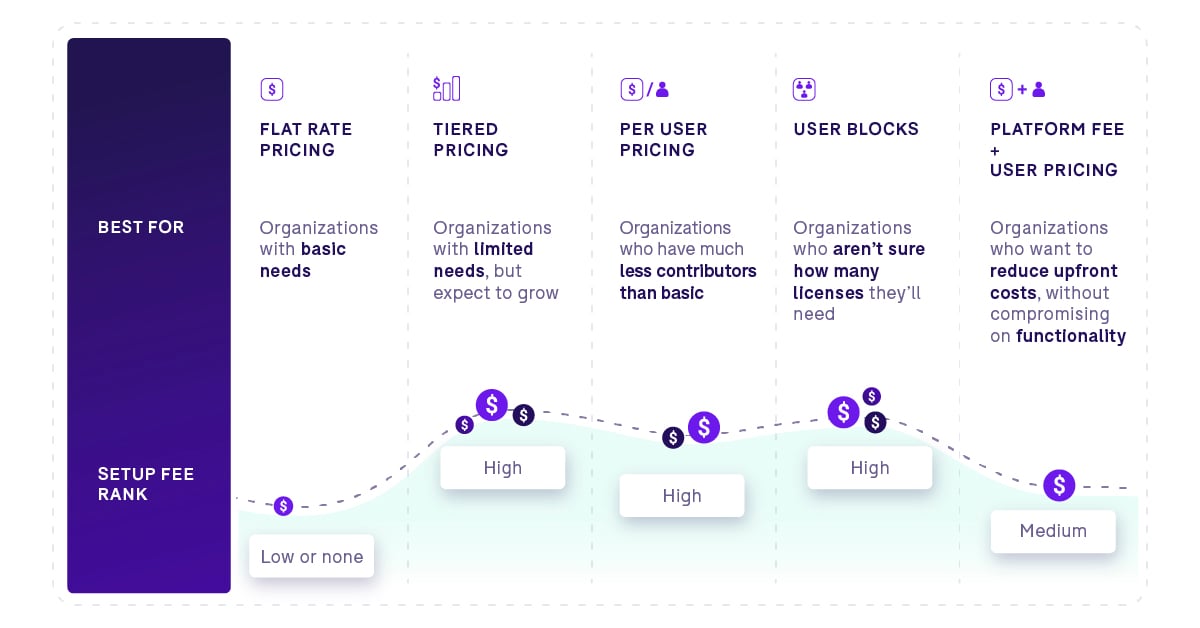
Most modern eQMS platforms are cloud-based and priced as a monthly subscription. Here are the models you will encounter.
- Flat rate pricing: One price, all functionality, all users. Simple and predictable, but systems using this model tend to be more basic.
- Best for: Smaller organizations with straightforward quality needs and a low total user count.
- Tiered or module-based pricing: You pay for the modules or functionality tiers you need, not the full platform.
- Best for: Organizations with limited needs today that want to avoid paying for features they will not use right away.
- Per user pricing: A monthly license fee per user, often with different license types for contributors versus read-only users.
- Best for: Organizations with a clear mix of active contributors and occasional users who only need read access.
- User block pricing: You purchase a block of licenses and pay based on active monthly users within that block.
- Best for: Organizations where user volume fluctuates or is difficult to predict at the time of signing.
- Platform fee plus user pricing: A monthly platform fee combined with per-user pricing. This model lowers upfront costs and gives you flexibility on user volume as your team grows.
- Best for: Scaling biotech and pharma organizations that want cost predictability on the platform side without locking into a fixed user count.
Is there a perfect pricing model?
No. Every organization's situation is different, and the right model depends on your team size, growth trajectory, and how your users interact with the system.
That said, for scaling life sciences organizations, the platform fee plus user pricing model tends to be the most practical starting point. It keeps costs predictable at the platform level while giving you flexibility on users as your team grows.
The ability to separate contributor licenses from read-only licenses also matters in a regulated environment, where not everyone needs full system access but everyone needs visibility into current SOPs and training requirements.
When evaluating pricing, think beyond the monthly subscription. Factor in implementation fees, migration costs, and what happens to your costs when you double your headcount in year two.
Here’s a quick guide to help you figure out which plan is best:
What to consider before implementing an eQMS?
Getting implementation right matters more than getting it done fast. Here are the key things to work through before you start.
Timelines
Some systems take longer to go live than others. A longer implementation is not a red flag. Validation, user acceptance testing, and configuration take time, and cutting corners here creates compliance risk later.
Before you sign, confirm what the implementation includes, how long validation takes, and what your team needs to do to get the system configured for the way you actually work.
Users
Before you evaluate any system, map out who will use it and how. Think about who authors SOPs, who approves them, who completes training, and who needs read-only visibility. This shapes which license types you need and whether the system's workflows match how your team operates.
Bring this clarity into your product demonstrations so you are evaluating real use cases, not a generic feature tour.
Business processes
Document how you manage quality today before you commit to a new system. Paper, SharePoint, Excel, or a point solution for SOPs and training, whatever your current setup, understand it first. Then evaluate where the new system improves those processes, and where it requires you to change them.
Moving to an eQMS is an opportunity to fix processes that were not working, not just digitize the ones that were. Do not translate a broken paper process into a broken electronic one.
Scope
Even a focused implementation affects more than one team. A new document control system touches everyone who authors, reviews, or trains on SOPs. Before you start, define what is in scope for this phase and what is planned for later. Identify which SOPs need to be revised to reflect the new system, and which teams outside of QA need to be informed or involved.
A clear scope prevents the project from expanding mid-implementation and keeps your go-live date on track.
Scale your organization and meet compliance demands
Quality issues surface at the worst possible moments when your team lacks real-time visibility into what is happening across your quality system. An eQMS gives your QA team the oversight to catch problems early, before they become inspection findings or trial delays.
Growth puts pressure on every manual process your organization relies on. The teams that scale without adding disproportionate headcount are the ones that put the right systems in place before the pressure hits.
We hope this guide gives you a clear starting point for evaluating and implementing an eQMS that fits where your organization is today and where it is headed.
About Montrium Quality Connect
Quality Connect is Montrium's eQMS built for life sciences organizations managing quality in regulated environments. The platform includes four modules: SOP Connect for document control, Training Connect for training management, CAPA Connect for quality event and corrective action management, and Change Connect for change control.
Each module works independently or as a fully integrated system, so you start with what you need today and expand as your organization grows. Quality Connect is cloud-based, validated for GxP environments, and built to meet 21 CFR Part 11 and Annex 11 requirements.





%20(1200%20x%20600%20px)%20(2)%20(1).png?width=2400&height=800&name=CTA%20-%20Blog%20(1200%20x%20400%20px)%20(1200%20x%20600%20px)%20(2)%20(1).png)




%20Share%20Best%20Practices%20for%20Improving%20TMF%20Quality.png)