
With constant stories surrounding the new financial rollercoaster that is Bitcoin, it’s not surprising that the technology underlying it and how those transactions are made is piquing interest in several major industries. “Blockchain”, an innovative method of sharing information, is the basis of Bitcoin—and all other cryptocurrencies—offering speed, security, and transparency to computerized data.
This peer-to-peer system may have originated with Bitcoin but it has become more and more apparent that there are many other usages for blockchain technology outside of online purchases and currency trading.
We too within the life sciences industry are looking to achieve similar goals, whether it be increasing the speed in which information is shared, increasing the level of security that is embedded in the management of information or the push for greater transparency. Is our industry also ripe for a transition to a blockchain-based way of operating? A recent study from IBM revealed that 16% of healthcare executives plan to put a commercial blockchain solution in place this year, while 56% expected to do so by 2020.
In the following article we explore what blockchain is, why it is a smart infrastructure choice for life sciences data in information, and how it could be used before, during, and after a clinical trial.
What exactly is Blockchain?
Let’s start by talking about blockchain in the context that most of us know, the financial sector. In a traditional financial transaction, a record of that transaction is held by a single party (a banking institution) in a document or system known as a ledger. This is usually a private record that is only visible to the record keeper.
Where Blockchain differs is that it takes this ledger public, creating transparency surrounding the transaction and making information available to all involved. Blockchain technology also utilizes a distributed computer network to create a database that stores timestamped transaction record. Every transaction—or record— in a given period of time, is recorded in what its creators call a “block.” After every transaction, that block is sent to all of the parties involved, and a “chain” of blocks is strung together in chronological order. This is the “blockchain”.
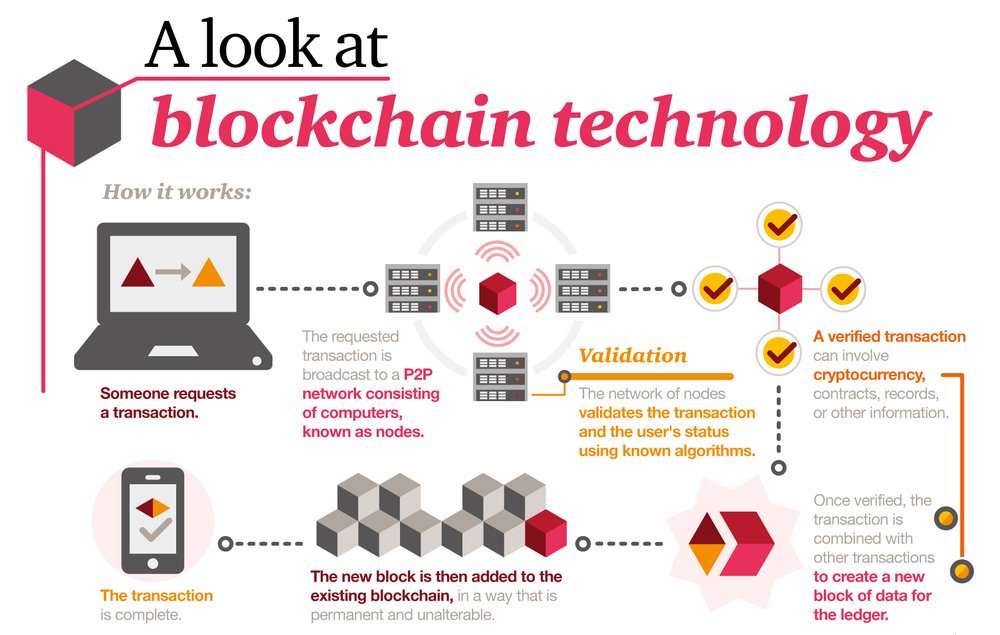
Image Source: https://www.digitalpulse.pwc.com.au/pwc-blockchain-infographic/
Everyone with access to the particular blockchain has access to all of the other information in the chain and its complete history. There is no single party that has complete control over the data and information, and instead, every party involved in the chain can verify the record of transactions without an intermediary.
How does Blockchain apply to the Exchange of Information?
In a classic model of information sharing, an intermediary was required to conduct a transaction. We placed full trust in these intermediaries and gave them full control of the information and data we sent.
In a blockchain-based system, this is eliminated entirely. Peer-to-peer transactions of encoded information are conducted across the entire network, with everyone involved having access to the encoded version of the data, without any single person moderating the transaction. While the transaction is open for all to see, anonymity as to who the transaction was conducted between and for what reason, is achieved by only allowing certain parties full access to the encrypted data. Kind of like an audit trail, accessible to parties involved in the audit trail.
How Can Blockchain be Incorporated into the Clinical Trials?
Within the conduct clinical trials, artifacts/documents like contracts, clinical protocols, and data sets make up the basis of how we interact and exchange information internally with peers, with the regulatory authorities, and with the consumers.
This information is crucial to everything relating to a clinical trial; recording experimental data, documenting regulatory compliance, and building data protection frameworks. However, the underlying technologies defining how these artifacts are exchanged and how these transactions are conducted are becoming antiquated (physical/electronic ledgers) if they even exist.
By thinking of trial data in a similar way as the financial data and looking at this data in the context of Bitcoin, blockchain offers a solution that could provide clinical teams with a way to provide internal visibility, improved consistency, and establish an immutable audit trail. Approaching data in this way could lay solid foundations for inspectors and auditors to view clinical trial information.
How does Blockchain Fit into Pre-Trial Activities?
Before a trial starts and patients are receiving treatment, there is a huge amount of data and documentation that needs to change hands before we even begin. How can Blockchain empower study startup and even pre-trial activities?
Collaboration
From day zero, clinical teams start collecting sensitive information – information that requires careful handling. This is particularly true for patient data, product information, and regulatory correspondences. However, collaboration is a major aspect of any trial design, with sites, clinical operations, quality, and regulatory affairs teams all needing to contribute to various documents before any work can start.
By moving to a blockchain model, data is automatically shared and easily accessed by anyone with access to the chain, creating a collaborative environment that tracks all versions of a document. For example, this is particularly useful when authoring protocols. As protocols can be lengthy documents with numerous revisions, blockchain offers monitoring and tracking of all changes made and all data on the contributing peer. As the chain needs to be maintained for all on the network, changes need approval from each user or “node” before it can be finalized.
Patient Consent Forms
Recruiting patients and having their express, fully informed consent is often one of the larger challenges during study startup. Without viewing the necessary documents, a patient wouldn’t be able to move forward and provide consent.
By creating a “smart contract” with Blockchain, consent becomes a truly patient driven process. The idea of an uninformed patient is a thing of the past. With patients’ being privy to the full chain of data at all times, as well as them being able to accept each piece of new information as it is finalized makes the entire process completely transparent and upfront.
How Can Blockchain Drive In-Trial Activities?
Once the trial “kicks-off” and patients are undergoing treatment, the amount of data being collected and shared is staggering. Currently, with single patient visits consisting of hundreds of data points spread across multiple sites, potentially across multiple countries, there is an incredible amount of data being created, and therefore there is enormous room for human error. How can blockchain impact the way clinical trials are conducted?
Collaboration/Disintermediation
While study startup is understandably a labor-intensive process and a vast amount of documentation is being created and distributed, the scale only expands as the study progresses and more activity takes place. Today, we measure how complete our TMFs and other repositories are through basic traffic light systems that, while valuable, only tell a small part of the story.
With Blockchain, we have the ability to share data to the chain in real-time, giving all participants in the study the ability to track progress beyond these traffic light systems that we see in current CTMS, eTMF and EDMS systems. All of the data you currently collect from monitoring visits, other trial stakeholders, IRBs & IECs and from the regulatory authorities can be tracked to its most granular level through the chain - creating a seamless mechanism where potential issues can be spotted almost immediately.
Without a centralized system or a middleman managing the transfer of data, information flows much quicker and isn’t caught at a bottleneck waiting for approvals. This cuts a significant amount of time away from trial management, making data immediately available for reporting and inspection.
Internet of Things (IoT)
With internet connected / web-enabled devices moving into almost all aspects of our daily lives, the idea of automated data gathering would be a colossal advancement. By bringing Internet of Things (IoT) devices into clinical trials we have the opportunity to drastically reduce completion times for key activities by allowing direct inputs into the trial system. By passing that quantitative information through a blockchain led process, this data is immediately made available to the entire clinical team in a way that makes it secure and error free, leaving no room for interpretation of notes or patient-self reports.
Similarly, this blockchain powered IoT network could reach much further than patient data. Integrating IoT reporting into the parameters of a smart contract—with a drug supplier for example—can allow you to constantly keep track of data you need to verify (example: drug transport and storage temperature). In this case, by maintaining a secure, immutable record of temperature you can be certain that at every point of the drug’s process, it was stored correctly and to protocol, enhancing trial validity and overall patient safety. Again, by having these blocks shared with all involved, it establishes a chain of evidence for remedying incidents and removing any opportunities for data tampering.
Patient Security
Patients involved in clinical trials are administered a range of different treatments and drug substances, some looking for an effect, others as a control. However, these highly controlled treatments need to be administered on both an accurate and a consistent basis, capturing these data-points as we conduct our analysis. If we were to introduce blockchain into this process, each data-point would be tracked and approved by everyone in the chain automatically, almost eliminating human error completely. Each participant in the chain can verify the drug administered as well as the data that comes with it.
Managing sensitive data
So much of what is recorded during a trial is sensitive. Whether it is patient data, company details, or trade secrets, it all needs to be kept secure to maintain patient confidentiality and intellectual property. In a traditional process, safeguarding data needs to be built into the company culture, however, as we have seen in the past, data breaches are more common than we would like, and at times even go unreported.
As we’ve mentioned before, Blockchain is built on an encrypted platform, securing documents and transactions to those who are authorized in the chain. This means that even if the data was intercepted by another device or person, nothing would be readable, which adds another layer of almost impenetrable security.
The other benefit of having a blockchain network model when managing sensitive data is if a system goes offline or becomes compromised. With all those in the chain having the entire chain available to them, if one system were to go down, or a device was lost, the other nodes (users) in the chain wouldn’t lose their data, as all nodes hold the entire chain. This means that as long as one node remains, the data is secure and like a school of fish, there is safety in numbers.
Working with and Moving Documents after the Trial
As we all know, once a trial is complete there’s a huge responsibility to complete reports and pass the data along to the sponsor and the regulators in an organized manner. As blockchain makes the document trail apparent, transparent and available to all, it’s an ideal technology for a regulated environment like the Life Sciences. How would blockchain help with study closeout?
Battling Fraudulent Data
It happens more often than we’d like to admit, but fraudulent data is something that challenges researchers during the course of clinical trials. It’s easy as a researcher to get personally invested in their work, leading some to make changes to data or “cherry picking” facts that support their initial conclusions. While this isn’t something we feel is widespread, it could lead to sanctions and issues should regulatory bodies expose these indecencies.
A blockchain driven process can prevent bias from working its way into the work. Since nothing can be altered or edited in a chain without all other parties approving these changes, it’s almost impossible to edit the record. For inspectors, or reviewers verifying data, this is a huge development, meaning that not only can they be certain of the veracity of the data and documentation, but they can look at the whole dataset and draw conclusions with a complete picture in front of them.
Financial Disintermediation
Getting back to bitcoin and the financial side of blockchain, money needs to change hands regularly in clinical trials. From sponsors to CROs, from CROs to Research Sites, and from Sites to Principle Investigators and patients, money needs to flow to make sure the business of research can be completed.
With all transactions in a chain being secured through encryption—only available to those with access—actual financial transactions can be managed in an extremely secure manner, removing the intermediary—an extra step that can add extra risk. By taking the financial middleman out of the equation, this could even lower trial costs and prove to be a more efficient way of doing business.
Key Takeaways
Blockchain provides a distinctive and innovative data infrastructure model with a whole range of benefits. By committing to passing information in encrypted blocks, teams could well see other benefits to using blockchain like increased team efficiency, better collaboration, and a reduction in overall study costs.
However, Blockchain—like any new technology—isn’t a be-all, end-all solution to solve all of our data management issues, but it does provide an interesting discussion point for IT leaders within clinical research. Will we see these leaders invest more in exploring the uses of blockchain in clinical research and the life sciences industry as a whole? Or will this be just another technology innovation that is perceived as too risky to implement while the rest of the world reaps the benefits?

Katherine Cianciarelli
As Product Owner, Katherine ensures that the Connect platform is aligned with life science customers' needs and requirements. An engineer by trade, she is a key member of Montrium's team, playing an active role in disseminating product feedback to the development team to build better products for our customers. Katherine regularly contributes articles to the Montrium blog and other publishers surrounding the changing regulatory landscape, IT transformation in life sciences and process optimization.














.png)