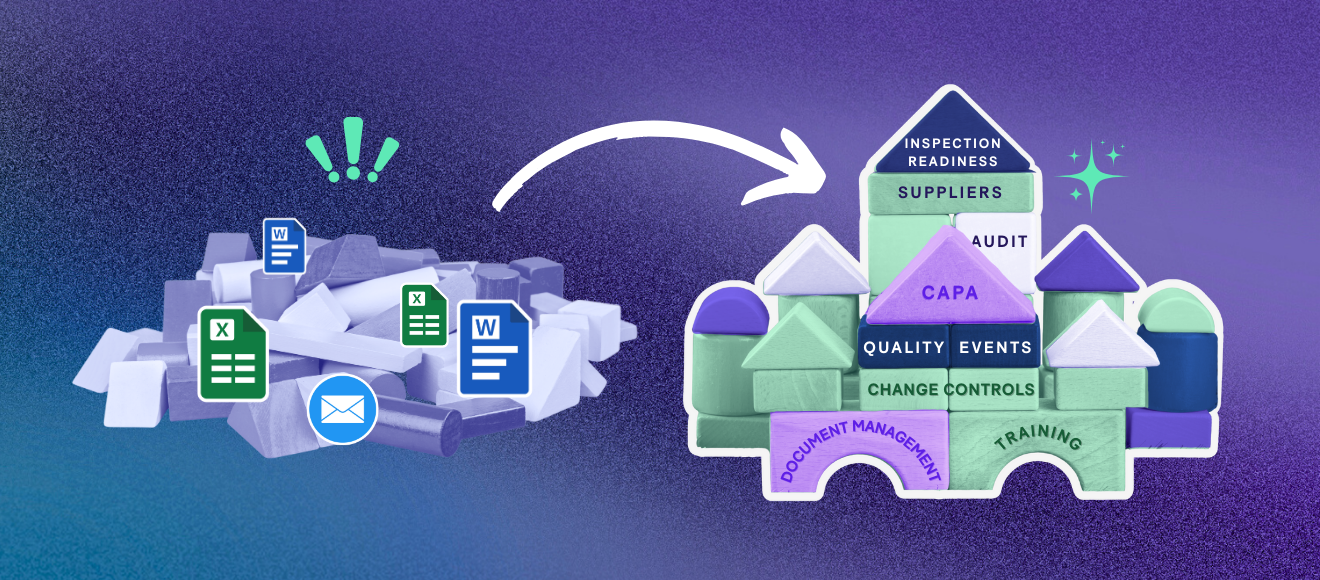
Look, we get it. You’re a lean biotech team trying to juggle clinical trials, investor decks, and an ever-growing pile of regulatory obligations. Your current QMS is probably a chaotic cocktail of Excel spreadsheets, SharePoint folders, and sheer willpower. And who could blame you?
But here's the thing: it doesn’t have to be this way. When implemented correctly, your QMS becomes an operational command centre. It ensures your processes, people, and products consistently meet standards from the likes of the FDA and ICH, saving you from costly and time-consuming R&D roadblocks down the line. Putting in place a right-sized QMS for biotech isn’t just a box to check for the regulators—it’s a cheat code to get your product to market faster.
Now that we’re on the same page about the why, let’s get into the how. In this article, we'll break QMS implementation into five manageable phases so small biopharma teams like yours can achieve inspection-ready digital control within a timeline (and budget) that works for you.
Why QMS Implementations Fail
Okay, the how is coming soon—we promise. But before we jump straight into QMS implementation steps, it’s helpful to understand some of the reasons why QMS projects crash and burn so that you can avoid them along your own journey. Spoiler alert: it's usually not the technology's fault.
1. Culture & Leadership Gaps
Here's a hard truth: if your leadership treats quality like a bothersome checkbox exercise, your QMS implementation is doomed before it starts. We've all seen leaders who love to talk about quality culture, but change their tune when quality processes slow down their "move fast and break things" mentality.
Real quality culture means leadership actually owns the vision, sets clear expectations, and—brace yourself—occasionally says no to shortcuts that would make investors happy but regulators furious.
2. Strategy Deficit
Too many companies fall into the pattern of reactivity, finding themselves so busy chasing the latest regulatory fire that they never step back to build a coherent strategy.
This reactive approach leads to a sort of "quality whack-a-mole”, if you will—you solve one compliance issue only to have three more pop up because you never addressed the root cause. Meanwhile, your team burns out from constantly being in crisis mode.
3. Software Mis-fit: The "Luxury Race Car" Problem
When it comes to building a QMS for biotech, you often don’t need a luxury race car—you just need a dependable sedan that can get you where you want to go.
Too many biotechs get seduced by the type of enterprise software that's built for Big Pharma with armies of quality professionals and unlimited budgets. You end up paying Ferrari prices for features you'll never use while your QA team of three people drowns in complexity.
4. Poor Data Integrity Controls
The FDA isn’t messing around when it tells people that all data need to be "attributable to the person generating the data, legible, contemporaneously recorded, original or a true copy, and accurate (ALCOA)".
Data integrity failures aren't just compliance headaches—they can undermine your entire regulatory strategy and tank investor confidence faster than you can say "FDA warning letter”.
What Makes a QMS Fit-for-Purpose
A QMS for biotech must walk the tightrope between regulatory compliance and operational sanity. Here's your checklist for building something that actually works:
✓ Align with ICH Q10 lifecycle model: ICH Q10 provides a comprehensive framework covering pharmaceutical development, manufacturing, and continuous improvement throughout the product lifecycle.
✓ Size to product phase: Your pre-clinical startup needs different QMS muscle than a Phase III company. Scale your system to match your regulatory risk profile, not your aspirations.
✓ Built-in data-integrity & cybersecurity layers: With FDA's emphasis on 21 CFR Part 11 requirements, your QMS needs bulletproof data controls from day one.
✓ Support real-time quality metrics & risk signals: Your QMS should be your early warning system, not just a document graveyard. Build in dashboards that actually help you prevent problems instead of just documenting them after the fact.
Pro-tip: Your module needs to change dramatically as you scale. Seed-stage companies typically need rock-solid document control and training management. Meanwhile, post-Series B companies need the full enterprise suite: supplier management, audit trails, complaint handling, and inspection readiness.
Choosing the Right Vendor
Listen, buying enterprise software can be a daunting task. Wading through a sea of product sheets to determine which features you need, fielding calls from salespeople, and reading proposals that will make your eyes water. Here are a few of our top tips to help you streamline the process and figure out how to choose QMS software that won't make you want to go back to Excel:
1. Product Maturity (aka GxP Street Cred
Does the software embed GxP best-practice workflows, or are you buying a generic platform with a marketing makeover? Real talk: if the sales demo includes phrases like "we can build that for you” more than a couple of times, run. You want best practices baked in, not custom development projects.
2.Ease of Use
User-friendliness determines whether your QMS is adopted or abandoned. If your quality system requires a PhD in software navigation, you’ll find your team reverting to Excel in short order. The best QMS should feel intuitive, not like you're operating mission control at NASA.
3. Validation
Does your vendor provide cloud validation packages and handle 21 CFR 11 shared responsibility models? Don't let yourself get stuck doing validation work that should be table stakes.
4. Implementation Model
Some vendors hand you software and ghost you faster than a bad date. Others provide templates, URS support, validation scripts, and hold your hand through go-live. Guess which approach leads to successful implementations?
Cloud vs On-Prem QMS
Let's settle this once and for all: when it comes to choosing a QMS for biotech, cloud-based is the way to go. Here’s why:
- Infrastructure: A lot of smaller companies simply don't have the infrastructure or resources to maintain an in-house eQMS. We're talking about needing a whole IT quality function with additional responsibilities for disaster recovery, upgrades, release requirements, system maintenance, and hosting. That's a lot of overhead for a lean biotech team.
- Scalability & Remote Access: It’s 2025, meaning that if you don’t already have remote team members, then you very well might in the near future. A cloud-based QMS allows stakeholders to access the system across timezones and geographies, setting you up for successful scaling down the road.
- Validation: When you find a vendor that can handle technical responsibilities like risk-based validation, it frees you up as the quality person (and your IT folks) to focus on actual value-added activities.
- Data Storage: If you don't have the right data storage processes and security features in place, you're just as vulnerable—if not more so—than hosting with a vendor that leverages professionally managed platforms like Microsoft Azure that have been vetted repeatedly for these concerns.
Quick decision tree: Do you have (1) unlimited budget, (2) dedicated IT infrastructure team, and (3) all users in one physical location? If you answered "no" to any of these, go cloud.
Building in Phases: QMS Implementation Steps That Actually Work
Rome wasn't built in a day, and neither should your QMS be. Smart biotechs take a phased approach that matches their regulatory priorities and resource constraints. Here are the QMS implementation steps that deliver value without breaking your team:
Phase 1 – Core Control
Document management + training (establish single source of truth)
This is your foundation—everything else crumbles without it. Get your SOPs, protocols, and training records under digital control. Stop the madness of "which version of this document is current?" and "did everyone get trained on the update?"
Phase 2 – Quality Events & Change Control
Change controls, quality events, CAPAs, effectiveness checks
Once your documents are locked down, tackle quality events. Build workflows that capture deviations quickly, track CAPAs to closure, and actually measure whether your corrective actions work.
Phase 3 – Supplier & Audit Management
Risk-based supplier oversight and audit trails
As you scale, audits and supplier quality become critical. Implement risk-based supplier qualification and monitoring that scales with your business.
Phase 4 – Complaints & Regulatory Inspection Readiness
Customer complaints and regulatory response
Commercial biotechs need robust complaint handling and the ability to respond quickly to regulatory inquiries. Build this before you need it.
Phase 5 – Continuous Improvement
Annual product quality reviews and continuous improvement initiatives
At the end of the implementation road is your continuous improvement initiatives and your annual product quality reviews—especially as you get closer to commercialization (unless you're already a commercial company, in which case this may change slightly). The key to all of this is dashboards that help you prevent problems instead of just documenting them. This is where your QMS transforms from compliance necessity to competitive advantage.
Important note: Many factors play into how you phase this out, but this is the typical approach most companies take.
From Planning to Monitoring
Your QMS implementation journey will be a long one, and it doesn’t stop at go-live. Here are some things to consider as you move along your QMS implementation roadmap:
- Strategy & Project Governance: Appoint a project lead (aka a Quality PM) and buffer your timelines. Everything takes longer than you think, especially when you're trying to maintain business-as-usual operations, so you need to plan realistically or you’ll pay the price in delays.
- Communication & Change Management: Change is hard, but you can make it less so. Plan training waves and hyper-care office hours from day one. Oh, and celebrate go-live wins. The best QMS in the world is worthless if your team isn’t keen to adopt it.
- Proactive Monitoring & KPIs: Build dashboards tracking CAPA cycle times, training compliance, and other metrics that align with the FDA's emphasis on risk-based surveillance and continuous improvement.
- Continuous-Improvement Loop: Annual product quality reviews and management review outputs should feed back into system improvements. You need formal structures for reviewing and continuously improving your quality system.
At the end of the day, you’ll find that much of your QMS implementation process comes down to one key tenet: failing to plan is planning to fail.
The Building Block Mindset
Here's the bottom line: QMS implementation doesn't have to be a death march. With the right building-block approach, you can transform from paper chaos to digital control without breaking your budget or your team's sanity.
Remember these key principles:
- Plan before you build (seriously, we can't stress this enough)
- Choose vendors that fit your culture and scale
- Take a phased approach that delivers value incrementally
- Build proactive monitoring from day one
- Think holistically about quality as everyone's responsibility
The regulatory bar is only going up, but the tech to meet it is better than ever. Build smart now, and you’ll scale faster and sleep better.
P.S. If you want more expert advice for implementing a quality management system, check out Montrium’s QMS Bootcamp–it’s free!







.png)
.png)

.png)




