Written by
Published on
October 2014

The Trial Master File (TMF) Reference Model is a supported initiative of the Drug Information Association’s (DIA) Document and Records Management Community and represents a single, unified interpretation of the regulations and best practices related to Trial Master Files that would be accepted by all clinical trial stakeholders, with a view to be adaptable and adopted by any organization.
The TMF Reference Model was created in 2010 based on feedback gained from a panel of industry stakeholders and continues to evolve under the auspices of the TMF Reference Model Steering Committee and extended team.
The TMF Reference Model Team is made up of over 350 representatives, from more than 170 biopharmaceutical companies, contract research organizations (CRO’s), consultants, technical vendors, industry groups, healthcare, academia and non-for-profit (NGO) organizations dedicated and seasoned life science professionals whose goal has been to identify what the true content of a standardized Trial Master File should be. Their objective in this has been to understand all of the essential documents that should individually and collectively permit the evaluation of the conduct of a trial and the quality of the data produced in accordance with regulatory requirements, industry opinion and best practices.
Organization of the TMF Reference Model
The TMF Reference Model consists of standard taxonomy and metadata and outlines the clear definition and organization of TMF content through the use of consistent nomenclature.
The Model consists of 4 main areas:
- Standard Contents – As defined by industry opinion on what is kept in a TMF
- Standard Naming – Based on ICH E6 Sect. 8 & industry-accepted terminology
- Standard Structure – To support paper and electronic systems
- Standard Metadata – For eTMF’s, minimum metadata at system and artifact level
The TMF Reference Model defines the various trial document types as artifacts, and labels them either as ‘core artifacts’, meaning they are required as part of the TMF per ICH guidelines or regulations, or as ‘recommended artifacts’ meaning that the artifact may not be produced, but if created or collected it is recommended to be included in the TMF. In addition, due to the vastness of industry terminology, alternate names (as relevant) and descriptions are supplied for each artifact.
The Trial Master File
(The Trial Master File by Eldin Rammell, Rammell Consulting)
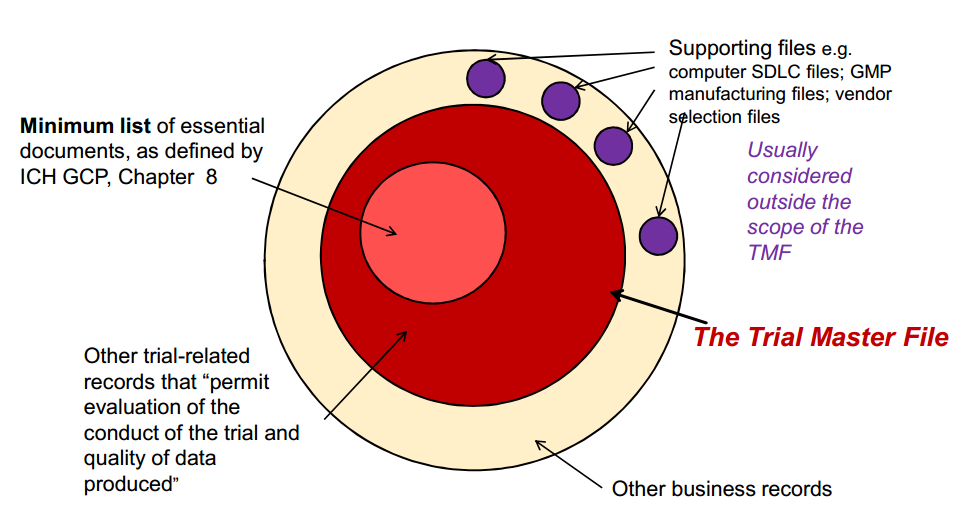
The artifacts have been organized into zones, where ‘like’ artifacts are grouped together. These zones are as follows:
- Trial Management
- Central Trial Documents
- Regulatory
- IRB/IEC and Other Approvals
- Site Management
- Investigational Product (IP) and Trial Supplies
- Safety Reporting
- Centralized Testing
- Third Parties
- Data Management
- Statistics
The Benefits of Adopting the TMF Reference Model
Through the implementation and use of the TMF Reference Model, organizations have the ability to significantly improve their TMF management processes, as well as incorporate a model that continues to gain global traction for the standardization of TMF Content.
As the industry continues to adopt the TMF Reference Model, it becomes easier to present TMF content to external parties such as inspectors and to transfer TMF content in a structured and more standard way between clinical trial stakeholders. This will be further reinforced as interoperability standards evolve between eTMF systems for content interchange.
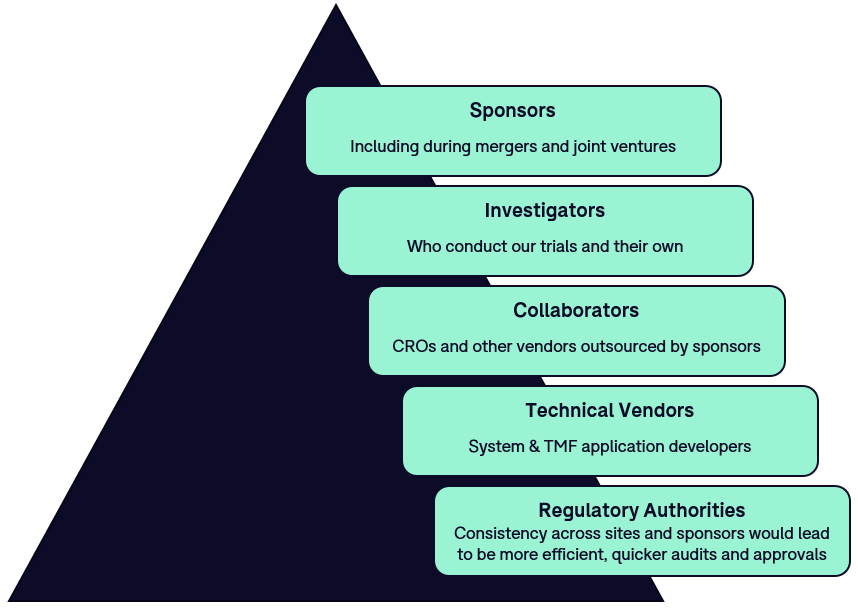
Who Benefits
The TMF Reference Model is a valuable tool for (as per the TMF Reference Model Website):
- Biopharmaceutical sponsor companies of any size, both commercial and institutional, involved in clinical trials.
- Clinical study team members, including trial and data management, clinical supplies, biostatistics, etc.
- Contract Research Organizations and vendors supporting TMFs.
- TMF Consultants.
- Site staff, including investigators and coordinators.
- Auditors and Regulators challenged with varying TMF terminology and file structures, which create inefficiency and a higher degree of variability during sponsor audits.
The Takeaway
The Model stands today as a reference for the industry and although not mandatory, it should be considered an opportunity for standardization across clinical research. The TMF Reference Model can be adapted to an electronic or paper TMF and does not endorse, nor, require, any specific technology or application.
Through the use of the TMF Reference Model, organizations could significantly simplify the administrative process across industry partnerships, and increase efficiency in collaborations between sponsors and CROs or acquisitions. The TMF Reference Model V3.2.0 was issued in November 2020. This is an industry initiative which is dependent on industry volunteers which is what makes it such a valuable resource. The Model continues to evolve based on collective industry knowledge and experience.
Useful Links:
- V3.2.0 of the TMF Reference Model
- V3.1.0 of the TMF Reference Model
- V2 of the TMF Reference Model
- V1 of the Framework for the Destruction of Paper
- TMF Reference Model LinkedIn Group
- TMF Reference Model Mailing List
Disclaimer: In accordance with the DIA Volunteer Code of Conduct, no volunteer shall use any information provided by the Association or acquired as a consequence of volunteer’s services to the Association in any manner other than in furtherance of his or her volunteer duties with DIA. Volunteers are expected to act at all times in the best interests of the Association and not for personal or third-party gain or financial enrichment. DIA reserves the right to reproduce, license, sell, display, and distribute copies of materials posted to the DIA website, in any medium or technology (including online) consistent with DIA’s non profit and tax exempt purposes.