
Stress is generally regarded as bad for us—mentally, physically, and emotionally. But sometimes, intentionally exposing ourselves to short-term stress can prove advantageous in the long run. We stomach the stress of a driving test in exchange for a lifetime of being free to operate a vehicle. We accept the stress brought on by university exams in order to reap the enduring benefits that a degree can bring to our careers. And, in the same vein, we should be conducting mock inspections so that we may navigate official inspections with far greater ease and avoid any unpleasant surprises.
When it comes to mock inspections, the devil is truly in the details. These exercises are large, interdepartmentally coordinated efforts that require outsize amounts of foresight and collaboration. Thus, planning them out properly is paramount. After 10 years of working in the life sciences industry, I’ve come to know a thing or two about how to plan mock inspections that actually deliver results. Today, I’ll be sharing some of that knowledge with you.
Below, we’ll go over how mock inspections can help your team and the concrete steps I would follow to plan the mock inspection of my dreams.
- What Are Mock Inspections and How Can They Help You?
- Part I: How I'd Plan the Mock Inspection of My Dreams
- Part II: How I'd Execute the Perfect Mock Inspection
- Part III: A Remediation Roadmap for Real Results
- Final Thoughts on Designing the Perfect Mock Inspection
What Are Mock Inspections and How Can They Help You?
Essentially, a mock inspection is a simulated version of an official inspection where you have the chance to run through all the motions and processes.
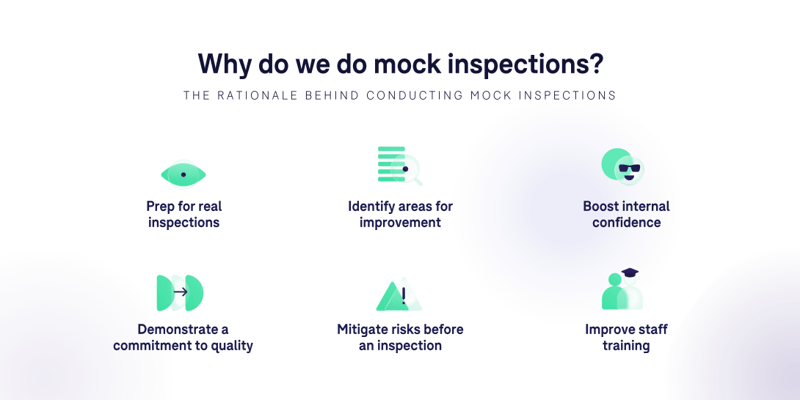
We all know that inspections send anxiety levels through the roof. So why would anyone want to subject themselves to this voluntarily? Well, like I said earlier, exposing yourself to short-term stress can bring significant long-term benefits. Running a mock inspection allows you to identify and remediate potential findings before a real inspector does, and also helps to acclimate your team to the inspection experience. This can be particularly useful for training new staff members and getting them up to speed on the procedural requirements of inspections. Of course, I could spend time going over the myriad of other benefits offered by mock inspections—you know, demonstrating a commitment to quality, mitigating risk, etc.—but I think you’ve already got the idea.
Now, let’s turn to the real raison d’être of this article: how to design the perfect mock inspection, or at least one that actually delivers results.
Part I: How I’d Plan the Mock Inspection of My Dreams
When designing a mock inspection, there are three main phases that you’ll need to address:
- Planning
- Execution
- Remediation
Let’s start from the beginning.
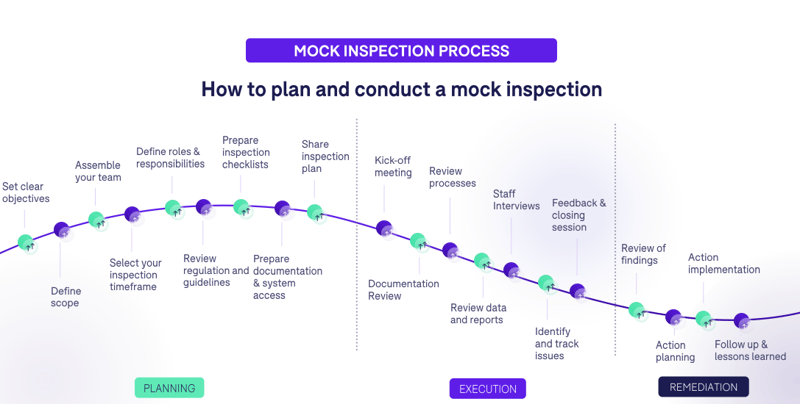
Planning a mock inspection can take months of dedicated work to pull off. There are dozens of steps, broken down into hundreds of sub-steps, all of which equate to tasks that must be completed by various departments and staff members. For the sake of your time, I’ve summarized the high-level steps that are involved in planning mock inspections.
The Planning Phase
- Set clear objectives: Define what you want to achieve with the mock inspection. This could be assessing compliance, identifying gaps, training staff, or preparing for an upcoming regulatory inspection.
- Define the scope: Specify which aspects of the clinical trial or process will be inspected. This might encompass specific sites, certain aspects of the TMF, or specific phases of a trial.
- Assemble your team: Gather a team of experts, which could include internal staff or external consultants. Ideally, they should have a mixture of clinical, regulatory, and quality assurance backgrounds.
- Select your inspection timeframe: Decide on the duration and dates of the mock inspection. This will help in logistics planning and ensuring the availability of necessary personnel and resources.
- Define roles and responsibilities: Assign specific tasks to team members. For instance, who will review documentation, who will conduct interviews, and who will manage the logistics?
- Review regulation and guidelines: Familiarize the team with the most current regulations and guidelines that pertain to the inspection. This ensures that the mock inspection is aligned with real-world standards. If you're in the US, for instance, you'll be performing a mock FDA inspection. Mock FDA inspections entail assessing your regulatory compliance with the applicable FDA regulations to prepare you for an actual FDA inspection, so make sure you've read up on the appropriate regulations.
- Prepare inspection checklists: Create or download structured lists of items to review, which will guide the inspection process and ensure comprehensiveness.
- Prepare documentation and system access: Ensure that all relevant documents are accessible and that the inspection team has access to any necessary systems or databases.
- Share inspection plan: Inform the relevant personnel about the upcoming mock inspection, its objectives, scope, and what is expected of them.
The above steps give you an overview of the factors I would take into consideration when architecting the perfect mock inspection. The planning phase is critical to the success of the exercise, and as such, it’s not something that I ever take lightly. Thoughtful, thorough planning will help to set you up for success in the next stage of the mock inspection roadmap: execution.
My advice: If you have the resources to do so, I recommend bringing in an external company or contractor to carry out the mock inspection. It’s much easier for someone outside of your team to remain unbiased and keep their faux inspector hat on at all times—two factors which are crucial to the success of the exercise.
Part II: How I’d Execute the Perfect Mock Inspection
Now, as I’m certain all life science professionals are painfully aware, even the most thorough of plans can quickly crumble to pieces under the pressure of an inspection. You think you have it all mapped out until an inspector arrives, and then all bets are off. Right about now, you may be wondering, “But wait, didn’t you just say that the planning phase is critical?” Yes, I did! And I stand by that. However, ensuring you understand how to properly execute a mock inspection—and by extension, a real inspection—is equally important.
With that being said, allow me to share the seven key steps I swear by for executing the perfect mock inspection.
The Execution Phase
- Kickoff meeting: Start with an initial meeting to set the tone, reiterate objectives, and align the team.
- Documentation review: Examine the TMF and other pertinent documents for compliance and completeness.
- Review processes: Evaluate the actual procedures being followed during the trial, ensuring they match with documented processes and meet regulatory requirements.
- Review data and reports: Examine the data collected in the trial and any interim or final reports to ensure accuracy and integrity.
- Staff interviews: Engage with clinical trial staff to assess their understanding of processes, protocols, and regulations. This can also help identify undocumented practices.
- Identify and track issues: As problems or gaps are found, document them clearly. Use a tracking system to ensure they are addressed in the remediation phase.
- Feedback and closing session: At the end of the mock inspection, provide feedback to the inspected party, summarizing findings and next steps.
These steps should give you a fairly good idea of the activities that you and your team will need to undertake during the course of a mock inspection. Once again, having a third party serve as the inspector for the exercise can ensure that the aforementioned review processes remain impartial and are able to unearth true areas for improvement. But what happens once you’ve actually discovered those areas for improvement? Well, that’s where the remediation phase comes in.
Part III: A Remediation Roadmap for Real Results
So, you’ve gone through your mock inspection exercise and you’ve discovered that you have several areas that you need to focus on if you want to mitigate potential findings. While it may sound counterintuitive, this is actually wonderful news. In this case, the mock inspection has done exactly what it was designed to do and will help you to take your inspection readiness to the next level—if you properly address the issues, that is. But how do you translate the results of the exercise into tangible improvements? You remediate, of course!
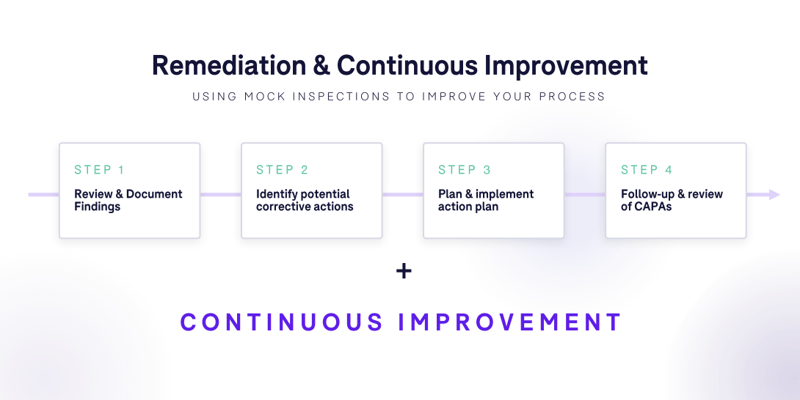
The Remediation Phase
- Review of findings: Take a detailed look at the issues identified during the execution phase, understanding the root causes.
- Action planning: Develop a structured plan and identify potential corrective actions to address each finding. This should include specific actions, responsibilities, and timelines.
- Action implementation: Execute the plan, ensuring that all identified issues are addressed.
- Follow-up & lessons learned: After remediation actions have been implemented, reassess to ensure effectiveness. Document lessons learned to improve future trials or inspections.
At the end of the day, remediation isn’t intended to chastise or punish, but rather to educate and develop future-proof processes to bolster inspection readiness.
Final Thoughts on Designing of the Perfect Mock Inspection
Before we wrap things up, I’d like to leave you with a little food for thought about the ROI of mock inspections. After having seen everything laid out above, you might be feeling daunted by the effort and costs that are required to perform a mock inspection. If that’s the case, I invite you to think about the cost of not performing a mock inspection—and potentially failing a real one. For me, it seems that the risk of not passing an official inspection far outweighs the short-term reward of not performing a mock inspection.
In my experience, mock inspections are an invaluable tool for organizations to proactively assess and fortify their compliance, procedures, and readiness for actual regulatory inspections. Through a thoughtfully designed and executed mock inspection process, spanning from planning to remediation, you can identify gaps, train staff, and ensure that you’re always a step ahead in maintaining the highest standards of clinical practice.
As for the idea of a “perfect” mock inspection, you may be wondering if one really does exist. My answer? Yes, but it looks different for everyone. What’s most important is that your mock inspection exercise is specifically tailored to the unique context and needs of your organization.

Christina Mantzioros
With a mandate to bridge the gap between clinical research, technical development, and business applications, Christina plays a vital role as Montrium's Product Implementation Manager. With over five years experience working in project management roles in clinical operations departments at both Academic and Clinical Research Organizations, Christina provides the Montrium team with essential insights into how clinical professionals use our products and platforms. Highly engaged with the Life Science industry and an expert on clinical and regulatory best practices, Christina regularly contributes to industry conferences, company webinars as well as the Montrium blog.










-1.png)
.png)

-1.png)