Electronic Trial Master Files (eTMFs) are one of the most widely discussed topics in clinical trials today, with a wealth of experts and specialists weighing in on the importance of good TMF management, best practices and the regulations. However, while the buzzword is getting more and more attention in the life sciences, it’s often difficult to understand exactly where the industry stands on eTMFs and the acceptance and growth of the technology.
- What is eTMF Software? And Does My Organization Need To Implement One?
- 12 of the Most Valuable eTMF Software Features You Should Be Looking For
- 10 Benefits TMF Managers are Achieving with eTMF Systems
As we begin to understand the impact eTMF systems have on the way we manage clinical trials, it’s also important to understand how the industry feels about eTMF solutions, and what the current state of eTMF is. Using research compiled through a recent ISR Report in 2016, as well as several other reports, research, and information, we’ve produced an infographic that compiles the key results of the current state of TMF management, focusing specifically on eTMF solutions.
The infographic is fun to read, and great to share with colleagues! In addition, some of the key stats in the article can act as a great starting point as you lobby for eTMF at your organization.
↓Check it out below↓
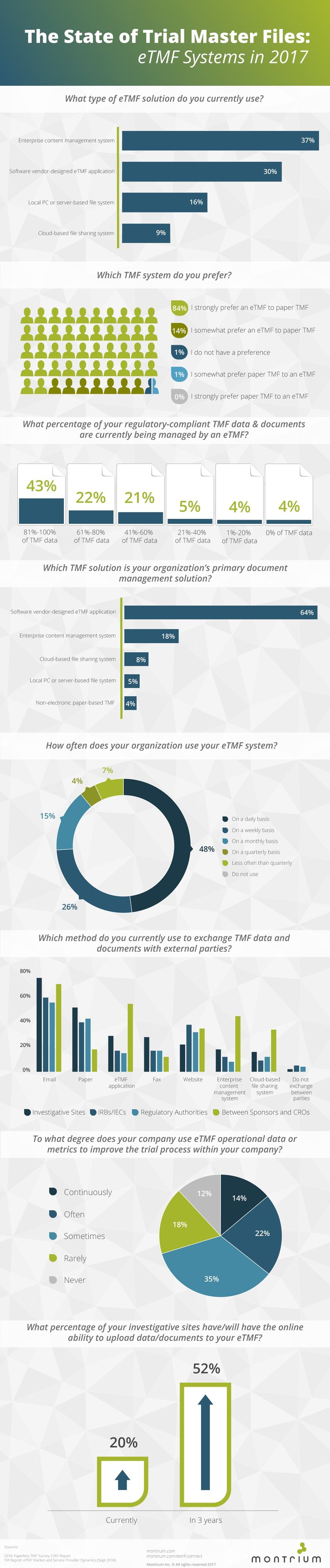
Here are some quick stats to tweet:
84% of companies strongly prefer an eTMF system to a paper TMF - Tweet This
43% of companies say that they store 81 to 100% of their TMF data and documents in an eTMF - Tweet This
64% of companies currently use an eTMF as their primary document management solution - Tweet This
In 3 years, 52% of investigative sites will have the ability to upload directly to the eTMF - Tweet This